Utility of the FebriDx point-of-care test for rapid triage and identification of possible coronavirus disease 2019 (COVID-19)
- PMID: 32896946
- PMCID: PMC7988545
- DOI: 10.1111/ijcp.13702
Utility of the FebriDx point-of-care test for rapid triage and identification of possible coronavirus disease 2019 (COVID-19)
Abstract
Objectives: The Coronavirus disease 2019 (COVID-19) pandemic is straining healthcare resources. Molecular testing turnaround time precludes having results at the point-of-care (POC) thereby exposing COVID-19/Non-COVID-19 patients while awaiting diagnosis. We evaluated the utility of a triage strategy including FebriDx, a 10-minute POC finger-stick blood test that differentiates viral from bacterial acute respiratory infection through detection of Myxovirus-resistance protein A (MxA) and C-reactive protein (CRP), to rapidly isolate viral cases requiring confirmatory testing.
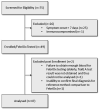
Methods: This observational, prospective, single-center study enrolled patients presenting to/within an acute care hospital in England with suspected COVID-19 between March and April 2020. Immunocompetent patients ≥16 years requiring hospitalisation with pneumonia or acute respiratory distress syndrome or influenza-like illness (fever and ≥1 respiratory symptom within 7 days of enrolment, or inpatients with new respiratory symptoms, fever of unknown cause or pre-existing respiratory condition worsening). The primary endpoint was diagnostic performance of FebriDx to identify COVID-19 as a viral infection; secondary endpoint was SARS-CoV-2 molecular test diagnostic performance compared with the reference standard COVID-19 Case Definition (molecular or antibody detection of SARS-CoV-2).
Results: Valid results were available for 47 patients. By reference standard, 35 had viral infections (34/35 COVID-19; 1/35 non-COVID-19; overall FebriDx viral sensitivity 97.1% (95%CI 83.3-99.9)). Of the COVID-19 cases, 34/34 were FebriDx viral positive (sensitivity 100%; 95%CI 87.4-100); 29/34 had an initial SARS-CoV-2 positive molecular test (sensitivity 85.3%; 95%CI 68.2-94.5). FebriDx was viral negative when the diagnosis was not COVID-19 and SARS-Cov-2 molecular test was negative (negative predictive value (NPV) 100% (13/13; 95%CI 71.7-100)) exceeding initial SARS-CoV-2 molecular test NPV 72.2% (13/19; 95%CI 46.4-89.3). The diagnostic specificity of FebriDx and initial SARS-CoV-2 molecular test was 100% (13/13; 95%CI 70-100 and 13/13; 95%CI 85.4-100, respectively).
Conclusions: FebriDx could be deployed as part of a reliable triage strategy for identifying symptomatic cases as possible COVID-19 in the pandemic.
© 2020 The Authors. International Journal of Clinical Practice published by John Wiley & Sons Ltd.
Conflict of interest statement
Authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Emergency Department point-of-care antiviral host response testing is accurate during periods of multiple respiratory virus co-circulation.J Infect. 2024 Jan;88(1):41-47. doi: 10.1016/j.jinf.2023.11.003. Epub 2023 Nov 15. J Infect. 2024. PMID: 37977337
-
Use of the FebriDx point-of-care test for the exclusion of SARS-CoV-2 diagnosis in a population with acute respiratory infection during the second (COVID-19) wave in Italy.Int J Infect Dis. 2021 Jul;108:231-236. doi: 10.1016/j.ijid.2021.04.065. Epub 2021 Apr 24. Int J Infect Dis. 2021. PMID: 33901656 Free PMC article.
-
Diagnostic accuracy of the FebriDx host response point-of-care test in patients hospitalised with suspected COVID-19.J Infect. 2020 Oct;81(4):607-613. doi: 10.1016/j.jinf.2020.06.051. Epub 2020 Jun 21. J Infect. 2020. PMID: 32579983 Free PMC article.
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2020 Sep 30;9:CD013639. doi: 10.1002/14651858.CD013639.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Nov 26;11:CD013639. doi: 10.1002/14651858.CD013639.pub3. PMID: 32997361 Updated.
-
Effectiveness of tests to detect the presence of SARS-CoV-2 virus, and antibodies to SARS-CoV-2, to inform COVID-19 diagnosis: a rapid systematic review.BMJ Evid Based Med. 2022 Feb;27(1):33-45. doi: 10.1136/bmjebm-2020-111511. Epub 2020 Oct 1. BMJ Evid Based Med. 2022. PMID: 33004426
Cited by
-
Performance of the FebriDx Rapid Point-of-Care Test for Differentiating Bacterial and Viral Respiratory Tract Infections in Patients with a Suspected Respiratory Tract Infection in the Emergency Department.J Clin Med. 2023 Dec 27;13(1):163. doi: 10.3390/jcm13010163. J Clin Med. 2023. PMID: 38202172 Free PMC article.
-
Developing a Tool for Differentiation Between Bacterial and Viral Respiratory Infections Using Myxovirus Resistance Protein A and C-Reactive Protein.Infect Dis Ther. 2024 Jan;13(1):105-119. doi: 10.1007/s40121-023-00901-2. Epub 2023 Dec 19. Infect Dis Ther. 2024. PMID: 38112973 Free PMC article.
-
Blood myxovirus resistance protein-1 measurement in the diagnostic work-up of suspected COVID-19 infection in the emergency department.Immun Inflamm Dis. 2022 Apr;10(4):e609. doi: 10.1002/iid3.609. Immun Inflamm Dis. 2022. PMID: 35349755 Free PMC article.
-
Utility of the FebriDx point-of-care assay in supporting a triage algorithm for medical admissions with possible COVID-19: an observational cohort study.BMJ Open. 2021 Aug 9;11(8):e049179. doi: 10.1136/bmjopen-2021-049179. BMJ Open. 2021. PMID: 34373308 Free PMC article.
-
Recent updates of interferon-derived myxovirus resistance protein A as a biomarker for acute viral infection.Eur J Med Res. 2024 Dec 23;29(1):612. doi: 10.1186/s40001-024-02221-8. Eur J Med Res. 2024. PMID: 39710743 Free PMC article. Review.
References
-
- Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high‐value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164:425‐434. - PubMed
-
- Kaplan EL, Top FH, Dudding BA, Wannamaker LW. Diagnosis of streptococcal pharyngitis: differentiation of active infection from the carrier state in the symptomatic child. J Infect Dis. 1971;123:490‐501. - PubMed
-
- The Centers for Disease Control and Prevention (CDC) . Evaluating and Testing Persons for Coronavirus Disease 2019 (COVID‐19). 2020. https://www.cdc.gov/coronavirus/2019‐ncov/hcp/clinical‐criteria.html. Accessed April 1, 2020.
-
- The European Centre for Disease Prevention and Control (ECDC) . Case definition for coronavirus disease 2019 (COVID‐19), as of 29 May 2020. 2020. https://www.ecdc.europa.eu/en/covid‐19/surveillance/case‐definition. Accessed May 29, 2020.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous