Cationic amphiphilic drugs as potential anticancer therapy for bladder cancer
- PMID: 32896947
- PMCID: PMC7718956
- DOI: 10.1002/1878-0261.12793
Cationic amphiphilic drugs as potential anticancer therapy for bladder cancer
Abstract
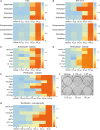
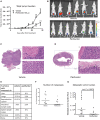
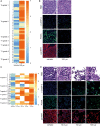
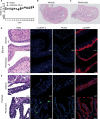
More effective therapy for patients with either muscle-invasive or high-risk non-muscle-invasive urothelial carcinoma of the bladder (UCB) is an unmet clinical need. For this, drug repositioning of clinically approved drugs represents an interesting approach. By repurposing existing drugs, alternative anticancer therapies can be introduced in the clinic relatively fast, because the safety and dosing of these clinically approved pharmacological agents are generally well known. Cationic amphiphilic drugs (CADs) dose-dependently decreased the viability of a panel of human UCB lines in vitro. CADs induced lysosomal puncta formation, a hallmark of lysosomal leakage. Intravesical instillation of the CAD penfluridol in an orthotopic mouse xenograft model of human UCB resulted in significantly reduced intravesical tumor growth and metastatic progression. Furthermore, treatment of patient-derived ex vivo cultured human UCB tissue caused significant partial or complete antitumor responses in 97% of the explanted tumor tissues. In conclusion, penfluridol represents a promising treatment option for bladder cancer patients and warrants further clinical evaluation.
Keywords: bladder cancer; cationic amphiphilic drugs; ex vivo culture; penfluridol; preclinical in vivo model.
© 2020 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Avritscher EB, Cooksley CD, Grossman HB, Sabichi AL, Hamblin L, Dinney CP & Elting LS (2006) Clinical model of lifetime cost of treating bladder cancer and associated complications. Urology 68, 549–553. - PubMed
-
- Alifrangis C, McGovern U, Freeman A, Powles T & Linch M (2019) Molecular and histopathology directed therapy for advanced bladder cancer. Nat Rev Urol 16, 465–483. - PubMed
-
- Sylvester RJ, van der Meijden A & Lamm Dl(2002) Intravesical bacillus Calmette‐Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta‐analysis of the published results of randomized clinical trials. J Urol 168, 1964–1970. - PubMed
-
- Madersbacher S, Hochreiter W, Burkhard F, Thalmann GN, Danuser H, Markwalder R & Studer UE (2003) Radical cystectomy for bladder cancer today – a homogeneous series without neoadjuvant therapy. J Clin Oncol 21, 690–696. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

