Representation of Older Adults in Cardiovascular Disease Trials Since the Inclusion Across the Lifespan Policy
- PMID: 32897289
- PMCID: PMC7489390
- DOI: 10.1001/jamainternmed.2020.2750
Representation of Older Adults in Cardiovascular Disease Trials Since the Inclusion Across the Lifespan Policy
Abstract
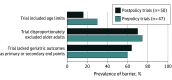
This review compares representation of older adults in interventional cardiovascular disease studies in the US before and after implementation of the National Institutes of Health’s Inclusion Across the Lifespan Policy.
Conflict of interest statement
Figures
Comment in
-
Inclusion Across the Lifespan in Cardiovascular Trials-A Long Road Ahead.JAMA Intern Med. 2020 Nov 1;180(11):1533-1534. doi: 10.1001/jamainternmed.2020.2735. JAMA Intern Med. 2020. PMID: 32897305 No abstract available.
References
-
- National Institutes of Health Revision: NIH Policy and Guidelines on the Inclusion of Individuals Across the Lifespan as Participants in Research Involving Human Subjects. National Institutes of Health; 2017. Notice NOT-OD-18-116. Accessed March 9, 2020. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-18-116.html