Outcomes of Intensive Systolic Blood Pressure Reduction in Patients With Intracerebral Hemorrhage and Excessively High Initial Systolic Blood Pressure: Post Hoc Analysis of a Randomized Clinical Trial
- PMID: 32897310
- PMCID: PMC7489424
- DOI: 10.1001/jamaneurol.2020.3075
Outcomes of Intensive Systolic Blood Pressure Reduction in Patients With Intracerebral Hemorrhage and Excessively High Initial Systolic Blood Pressure: Post Hoc Analysis of a Randomized Clinical Trial
Abstract
Importance: The safety and efficacy of intensive systolic blood pressure reduction in patients with intracerebral hemorrhage who present with systolic blood pressure greater than 220 mm Hg appears to be unknown.
Objective: To evaluate the differential outcomes of intensive (goal, 110-139 mm Hg) vs standard (goal, 140-179 mm Hg) systolic blood pressure reduction in patients with intracerebral hemorrhage and initial systolic blood pressure of 220 mm Hg or more vs less than 220 mm Hg.
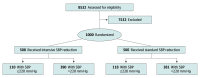
Design, setting, and participants: This post hoc analysis of the Antihypertensive Treatment of Acute Cerebral Hemorrhage-II trial was performed in November 2019 on data from the multicenter randomized clinical trial, which was conducted between May 2011 to September 2015. Patients with intracerebral hemorrhage and initial systolic blood pressure of 180 mm Hg or more, randomized within 4.5 hours after symptom onset, were included.
Interventions: Intravenous nicardipine infusion titrated to goals.
Main outcomes and measures: Neurological deterioration and hematoma expansion within 24 hours and death or severe disability at 90 days, plus kidney adverse events and serious adverse events until day 7 or hospital discharge.
Results: A total of 8532 patients were screened, and 999 individuals (mean [SD] age, 62.0 [13.1] years; 620 men [62.0%]) underwent randomization and had an initial SBP value. Among 228 participants with initial systolic blood pressures of 220 mm Hg or more, the rate of neurological deterioration within 24 hours was higher in those who underwent intensive (vs standard) systolic blood pressure reduction (15.5% vs 6.8%; relative risk, 2.28 [95% CI, 1.03-5.07]; P = .04). The rate of death and severe disability (39.0% vs 38.4%; relative risk, 1.02 [95% CI, 0.73-1.78]; P = .92) was not significantly different between the 2 groups. There was a significantly higher rate of kidney adverse events in participants randomized to intensive systolic blood pressure reduction (13.6% vs 4.2%; relative risk, 3.22 [95% CI, 1.21-8.56]; P = .01), but no difference was observed in the rate of kidney serious adverse events.
Conclusions and relevance: The higher rate of neurological deterioration within 24 hours associated with intensive treatment in patients with intracerebral hemorrhage and initial systolic blood pressure of 220 mm Hg or more, without any benefit in reducing hematoma expansion at 24 hours or death or severe disability at 90 days, warrants caution against generalization of recommendations for intensive systolic blood pressure reduction.
Conflict of interest statement
Figures
Comment in
-
Intensive Blood Pressure Reduction in Patients With Intracerebral Hemorrhage and Extreme Initial Hypertension: Primum Non Nocere.JAMA Neurol. 2020 Nov 1;77(11):1351-1352. doi: 10.1001/jamaneurol.2020.3081. JAMA Neurol. 2020. PMID: 32897308 No abstract available.
-
Insights on Intensive vs Nonintensive Prerandomization Systolic Blood Pressure Reduction.JAMA Neurol. 2021 May 1;78(5):619-620. doi: 10.1001/jamaneurol.2021.0258. JAMA Neurol. 2021. PMID: 33720277 No abstract available.
-
Insights on Intensive Vs Nonintensive Prerandomization Systolic Blood Pressure Reduction-Reply.JAMA Neurol. 2021 May 1;78(5):620. doi: 10.1001/jamaneurol.2021.0261. JAMA Neurol. 2021. PMID: 33720302 No abstract available.
References
-
- Hemphill JC III, Greenberg SM, Anderson CS, et al. ; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology . Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032-2060. doi: 10.1161/STR.0000000000000069 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical