Favorable outcomes of COVID-19 in recipients of hematopoietic cell transplantation
- PMID: 32897885
- PMCID: PMC7685738
- DOI: 10.1172/JCI141777
Favorable outcomes of COVID-19 in recipients of hematopoietic cell transplantation
Abstract
BACKGROUNDUnderstanding outcomes and immunologic characteristics of cellular therapy recipients with SARS-CoV-2 is critical to performing these potentially life-saving therapies in the COVID-19 era. In this study of recipients of allogeneic (Allo) and autologous (Auto) hematopoietic cell transplant and CD19-directed chimeric antigen receptor T cell (CAR T) therapy at Memorial Sloan Kettering Cancer Center, we aimed to identify clinical variables associated with COVID-19 severity and assess lymphocyte populations.METHODSWe retrospectively investigated patients diagnosed between March 15, 2020, and May 7, 2020. In a subset of patients, lymphocyte immunophenotyping, quantitative real-time PCR from nasopharyngeal swabs, and SARS-CoV-2 antibody status were available.RESULTSWe identified 77 patients with SARS-CoV-2 who were recipients of cellular therapy (Allo, 35; Auto, 37; CAR T, 5; median time from cellular therapy, 782 days; IQR, 354-1611 days). Overall survival at 30 days was 78%. Clinical variables significantly associated with the composite endpoint of nonrebreather or higher oxygen requirement and death (n events = 25 of 77) included number of comorbidities (HR 5.41, P = 0.004), infiltrates (HR 3.08, P = 0.032), and neutropenia (HR 1.15, P = 0.04). Worsening graft-versus-host disease was not identified among Allo recipients. Immune profiling revealed reductions and rapid recovery in lymphocyte populations across lymphocyte subsets. Antibody responses were seen in a subset of patients.CONCLUSIONIn this series of Allo, Auto, and CAR T recipients, we report overall favorable clinical outcomes for patients with COVID-19 without active malignancy and provide preliminary insights into the lymphocyte populations that are key for the antiviral response and immune reconstitution.FUNDINGNIH grant P01 CA23766 and NIH/National Cancer Institute grant P30 CA008748.
Keywords: COVID-19; Stem cell transplantation; T cells; Transplantation.
Conflict of interest statement
Figures

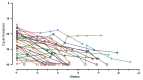
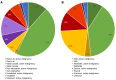




References
-
- Johns Hopkins Coronavirus Resource Center. COVID-19 United States Cases by County. https://coronavirus.jhu.edu/us-map Accessed Jun 2, 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

