Plasmodium sporozoites induce regulatory macrophages
- PMID: 32898164
- PMCID: PMC7500643
- DOI: 10.1371/journal.ppat.1008799
Plasmodium sporozoites induce regulatory macrophages
Abstract
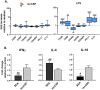
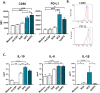
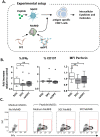
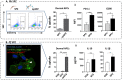
Professional antigen-presenting cells (APCs), like macrophages (Mϕs) and dendritic cells (DCs), are central players in the induction of natural and vaccine-induced immunity to malaria, yet very little is known about the interaction of SPZ with human APCs. Intradermal delivery of whole-sporozoite vaccines reduces their effectivity, possibly due to dermal immunoregulatory effects. Therefore, understanding these interactions could prove pivotal to malaria vaccination. We investigated human APC responses to recombinant circumsporozoite protein (recCSP), SPZ and anti-CSP opsonized SPZ both in monocyte derived MoDCs and MoMϕs. Both MoDCs and MoMϕs readily took up recCSP but did not change phenotype or function upon doing so. SPZ are preferentially phagocytosed by MoMϕs instead of DCs and phagocytosis greatly increased after opsonization. Subsequently MoMϕs show increased surface marker expression of activation markers as well as tolerogenic markers such as Programmed Death-Ligand 1 (PD-L1). Additionally they show reduced motility, produce interleukin 10 and suppressed interferon gamma (IFNγ) production by antigen specific CD8+ T cells. Importantly, we investigated phenotypic responses to SPZ in primary dermal APCs isolated from human skin explants, which respond similarly to their monocyte-derived counterparts. These findings are a first step in enhancing our understanding of pre-erythrocytic natural immunity and the pitfalls of intradermal vaccination-induced immunity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- [Internet] WHO: Geneva Switzerland, 19 November 2018, World Malaria report 2018. https://www.who.int/malaria/media/world-malaria-report-2018/en/.
-
- Seder R.A., et al., Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science, 2013. 341(6152): p. 1359–65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

