The p150 Isoform of ADAR1 Blocks Sustained RLR signaling and Apoptosis during Influenza Virus Infection
- PMID: 32898178
- PMCID: PMC7500621
- DOI: 10.1371/journal.ppat.1008842
The p150 Isoform of ADAR1 Blocks Sustained RLR signaling and Apoptosis during Influenza Virus Infection
Abstract
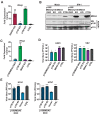
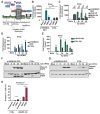
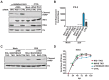
Signaling through retinoic acid inducible gene I (RIG-I) like receptors (RLRs) is tightly regulated, with activation occurring upon sensing of viral nucleic acids, and suppression mediated by negative regulators. Under homeostatic conditions aberrant activation of melanoma differentiation-associated protein-5 (MDA5) is prevented through editing of endogenous dsRNA by RNA editing enzyme Adenosine Deaminase Acting on RNA (ADAR1). In addition, ADAR1 is postulated to play pro-viral and antiviral roles during viral infections that are dependent or independent of RNA editing activity. Here, we investigated the importance of ADAR1 isoforms in modulating influenza A virus (IAV) replication and revealed the opposing roles for ADAR1 isoforms, with the nuclear p110 isoform restricting versus the cytoplasmic p150 isoform promoting IAV replication. Importantly, we demonstrate that p150 is critical for preventing sustained RIG-I signaling, as p150 deficient cells showed increased IFN-β expression and apoptosis during IAV infection, independent of RNA editing activity. Taken together, the p150 isoform of ADAR1 is important for preventing sustained RIG-I induced IFN-β expression and apoptosis during viral infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
The RNA-editing enzyme ADAR1: a regulatory hub that tunes multiple dsRNA-sensing pathways.Int Immunol. 2023 Mar 14;35(3):123-133. doi: 10.1093/intimm/dxac056. Int Immunol. 2023. PMID: 36469491
-
RNA editing at a limited number of sites is sufficient to prevent MDA5 activation in the mouse brain.PLoS Genet. 2021 May 13;17(5):e1009516. doi: 10.1371/journal.pgen.1009516. eCollection 2021 May. PLoS Genet. 2021. PMID: 33983932 Free PMC article.
-
RNA-Editing Enzyme ADAR1 p150 Isoform Is Critical for Germinal Center B Cell Response.J Immunol. 2022 Sep 15;209(6):1071-1082. doi: 10.4049/jimmunol.2200149. Epub 2022 Aug 17. J Immunol. 2022. PMID: 35977796
-
Deciphering the Biological Significance of ADAR1-Z-RNA Interactions.Int J Mol Sci. 2021 Oct 23;22(21):11435. doi: 10.3390/ijms222111435. Int J Mol Sci. 2021. PMID: 34768866 Free PMC article. Review.
-
ADAR1: "Editor-in-Chief" of Cytoplasmic Innate Immunity.Front Immunol. 2019 Jul 25;10:1763. doi: 10.3389/fimmu.2019.01763. eCollection 2019. Front Immunol. 2019. PMID: 31404141 Free PMC article. Review.
Cited by
-
Host Innate Antiviral Response to Influenza A Virus Infection: From Viral Sensing to Antagonism and Escape.Pathogens. 2024 Jul 3;13(7):561. doi: 10.3390/pathogens13070561. Pathogens. 2024. PMID: 39057788 Free PMC article. Review.
-
CRISPR editing of candidate host factors that impact influenza A virus infection.Microbiol Spectr. 2025 Mar 4;13(3):e0262724. doi: 10.1128/spectrum.02627-24. Epub 2025 Jan 31. Microbiol Spectr. 2025. PMID: 39887213 Free PMC article.
-
A-to-I RNA editing by ADAR and its therapeutic applications: From viral infections to cancer immunotherapy.Wiley Interdiscip Rev RNA. 2023 Sep 17:e1817. doi: 10.1002/wrna.1817. Online ahead of print. Wiley Interdiscip Rev RNA. 2023. PMID: 37718249 Free PMC article. Review.
-
ADAR1 p150 prevents HSV-1 from triggering PKR/eIF2α-mediated translational arrest and is required for efficient viral replication.PLoS Pathog. 2025 Apr 8;21(4):e1012452. doi: 10.1371/journal.ppat.1012452. eCollection 2025 Apr. PLoS Pathog. 2025. PMID: 40198737 Free PMC article.
-
Epigenetic Restriction Factors (eRFs) in Virus Infection.Viruses. 2024 Jan 25;16(2):183. doi: 10.3390/v16020183. Viruses. 2024. PMID: 38399958 Free PMC article. Review.
References
-
- Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5'-phosphates. Science. 2006;314(5801):997–1001. - PubMed
-
- Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, et al. 5'-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314(5801):994–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

