Intrahepatic CXCL10 is strongly associated with liver fibrosis in HIV-Hepatitis B co-infection
- PMID: 32898182
- PMCID: PMC7521747
- DOI: 10.1371/journal.ppat.1008744
Intrahepatic CXCL10 is strongly associated with liver fibrosis in HIV-Hepatitis B co-infection
Abstract
In HIV-hepatitis B virus (HBV) co-infection, adverse liver outcomes including liver fibrosis occur at higher frequency than in HBV-mono-infection, even following antiretroviral therapy (ART) that suppresses both HIV and HBV replication. To determine whether liver disease was associated with intrahepatic or circulating markers of inflammation or burden of HIV or HBV, liver biopsies and blood were collected from HIV-HBV co-infected individuals (n = 39) living in Bangkok, Thailand and naïve to ART. Transient elastography (TE) was performed. Intrahepatic and circulating markers of inflammation and microbial translocation were quantified by ELISA and bead arrays and HIV and HBV infection quantified by PCR. Liver fibrosis (measured by both transient elastography and liver biopsy) was statistically significantly associated with intrahepatic mRNA for CXCL10 and CXCR3 using linear and logistic regression analyses adjusted for CD4 T-cell count. There was no evidence of a relationship between liver fibrosis and circulating HBV DNA, qHBsAg, plasma HIV RNA or circulating cell-associated HIV RNA or DNA. Using immunohistochemistry of liver biopsies from this cohort, intrahepatic CXCL10 was detected in hepatocytes associated with inflammatory liver infiltrates in the portal tracts. In an in vitro model, we infected an HBV-infected hepatocyte cell line with HIV, followed by interferon-γ stimulation. HBV-infected cells lines produced significantly more CXCL10 than uninfected cells lines and this significantly increased in the presence of an increasing multiplicity of HIV infection. Conclusion: Enhanced production of CXCL10 following co-infection of hepatocytes with both HIV and HBV may contribute to accelerated liver disease in the setting of HIV-HBV co-infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


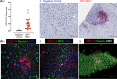


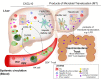
References
-
- Boyd A, Gozlan J, Maylin S, Delaugerre C, Peytavin G, Girard PM, et al. Persistent viremia in human immunodeficiency virus/hepatitis B coinfected patients undergoing long-term tenofovir: Virological and clinical implications. Hepatology (Baltimore, Md). 2014;60(2):497–507. Epub 2014/04/23. 10.1002/hep.27182 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

