Outgrowth of erlotinib-resistant subpopulations recapitulated in patient-derived lung tumor spheroids and organoids
- PMID: 32898185
- PMCID: PMC7478813
- DOI: 10.1371/journal.pone.0238862
Outgrowth of erlotinib-resistant subpopulations recapitulated in patient-derived lung tumor spheroids and organoids
Abstract
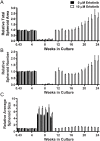
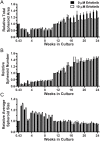
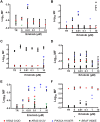
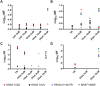
A model that recapitulates development of acquired therapeutic resistance is needed to improve oncology drug development and patient outcomes. To achieve this end, we established methods for the preparation and growth of spheroids from primary human lung adenocarcinomas, including methods to culture, passage, monitor growth, and evaluate changes in mutational profile over time. Primary lung tumor spheroids were cultured in Matrigel® with varying concentrations of erlotinib, a small molecule kinase inhibitor of epidermal growth factor receptor (EGFR) that is ineffective against KRAS mutant cells. Subtle changes in spheroid size and number were observed within the first two weeks of culture. Spheroids were cultured for up to 24 weeks, during which time interactions between different cell types, movement, and assembly into heterogeneous organoid structures were documented. Allele-specific competitive blocker PCR (ACB-PCR) was used to quantify low frequency BRAF V600E, KRAS G12D, KRAS G12V, and PIK3CA H1047R mutant subpopulations in tumor tissue residue (TR) samples and cultured spheroids. Mutant subpopulations, including multiple mutant subpopulations, were quite prevalent. Twelve examples of mutant enrichment were found in eight of the 14 tumors analyzed, based on the criteria that a statistically-significant increase in mutant fraction was observed relative to both the TR and the no-erlotinib control. Of the mutants quantified in erlotinib-treated cultures, PIK3CA H1047 mutant subpopulations increased most often (5/14 tumors), which is consistent with clinical observations. Thus, this ex vivo lung tumor spheroid model replicates the cellular and mutational tumor heterogeneity of human lung adenocarcinomas and can be used to assess the outgrowth of mutant subpopulations. Spheroid cultures with characterized mutant subpopulations could be used to investigate the efficacy of lung cancer combination therapies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

