Sustained Responses of Neutralizing Antibodies Against Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in Recovered Patients and Their Therapeutic Applicability
- PMID: 32898238
- PMCID: PMC7499518
- DOI: 10.1093/cid/ciaa1345
Sustained Responses of Neutralizing Antibodies Against Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in Recovered Patients and Their Therapeutic Applicability
Abstract
Background: Zoonotic coronaviruses have emerged as a global threat by causing fatal respiratory infections. Given the lack of specific antiviral therapies, application of human convalescent plasma retaining neutralizing activity could be a viable therapeutic option that can bridges this gap.
Methods: We traced antibody responses and memory B cells in peripheral blood collected from 70 recovered Middle East respiratory syndrome coronavirus (MERS-CoV) patients for 3 years after the 2015 outbreak in South Korea. We also used a mouse infection model to examine whether the neutralizing activity of collected sera could provide therapeutic benefit in vivo upon lethal MERS-CoV challenge.
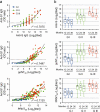
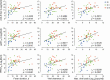
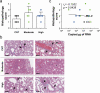
Results: Anti-spike-specific IgG responses, including neutralizing activity and antibody-secreting memory B cells, persisted for up to 3 years, especially in MERS patients who suffered from severe pneumonia. Mean antibody titers gradually decreased annually by less than 2-fold. Levels of antibody responses were significantly correlated with fever duration, viral shedding periods, and maximum viral loads observed during infection periods. In a transgenic mice model challenged with lethal doses of MERS-CoV, a significant reduction in viral loads and enhanced survival was observed when therapeutically treated with human plasma retaining a high neutralizing titer (> 1/5000). However, this failed to reduce pulmonary pathogenesis, as revealed by pathological changes in lungs and initial weight loss.
Conclusions: High titers of neutralizing activity are required for suppressive effect on the viral replication but may not be sufficient to reduce inflammatory lesions upon fatal infection. Therefore, immune sera with high neutralizing activity must be carefully selected for plasma therapy of zoonotic coronavirus infection.
Keywords: MERS-CoV; neutralizing antibody; plasma therapy.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

