Pharmacometric modeling to explore 4F-PCC dosing strategies for VKA reversal in patients with INR below 2
- PMID: 32898246
- PMCID: PMC7479946
- DOI: 10.1182/bloodadvances.2020002267
Pharmacometric modeling to explore 4F-PCC dosing strategies for VKA reversal in patients with INR below 2
Abstract
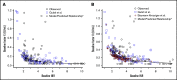
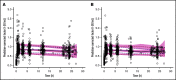
The indicated dose of 4-factor prothrombin complex concentrate (4F-PCC) for urgent vitamin K antagonist (VKA) reversal in patients with an international normalized ratio (INR) of 2 to 4 is 25 IU/kg, but there is no indicated dose for INR <2. We explored 4F-PCC dosing strategies for baseline INR <2. Clinical trial data were used to develop pharmacometric models for Factor X (FX) and FII, accounting for covariates including baseline INR. FX and FII levels over time were simulated for mean baseline INR levels of the clinical trial participants plus baseline INRs 3.1, 1.9, and 1.6. For each INR, 200 virtual male patients were simulated to evaluate 4F-PCC doses of 35, 25, 20, 15, 12.5, and 10 IU/kg. Given an elevated bleeding risk with VKA therapy in Japanese vs Western populations, results were stratified by Japanese and non-Japanese patients. Target levels of FX and FII were ≥50% activity at 30 minutes after dosing in ≥80% of patients. FX- and FII-time models were developed with 1088 FX observations from 193 patients and 1074 FII observations from 192 patients. Model-based simulations indicated that at baseline INR 3.1, ≥80% of patients achieved ≥50% FX and FII activity with 25 IU/kg and 20 IU/kg 4F-PCC, respectively; at baseline INR 1.9, corresponding doses were 20 IU/kg and 15 IU/kg 4F-PCC, and at baseline INR 1.6, corresponding doses were 15 IU/kg, and 10 IU/kg 4F-PCC. Trends in Japanese and non-Japanese patients were similar. In conclusion, low 4F-PCC doses (15-20 IU/kg) may be sufficient to achieve hemostatic levels of FX and FII in Japanese and non-Japanese patients with baseline INR <2.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: R.S. served as a consultant for CSL Behring, Octapharma, and Portola and received institutional research funding from Siemens. M.A.T. and A.M. are employees of CSL Behring. M.P. is an employee of Certara. A.C. reports that his institution has received research funding on his behalf from Alexion, Bayer, Bioverativ, Novo Nordisk, Pfizer, Shire, Spark, and Syntimmune and that he has served as a consultant for Genzyme, Kedrion, and Synergy. M.Y. has received lecture fees from Bayer, Bristol-Myers Squibb, and Daiichi-Sankyo. K.F. reports that his institution has received research funding on his behalf from Baxalta/Shire, Bayer, Pfizer, CSL Behring, Novo Nordisk, Biogen/Bioverativ, Kaketsuken/KM Biologics, Japan Blood Products Organization, and Chugai Pharmaceutical; he has served as a consultant for Baxalta/Shire, Bayer, CSL Behring, Novo Nordisk, Biogen/Bioverativ, Kaketsuken/KM Biologics, Chugai Pharmaceutical, SRL Inc., LSI Medience, Sekisui Medical, and Sysmex; and he has served as a speaker for Baxalta/Shire, Bayer, Pfizer, CSL Behring, Novo Nordisk, Biogen/Bioverativ, Kaketsuken/KM Biologics, Chugai Pharmaceutical, Siemens, Sekisui Medical, Fujirebio Inc., Torii Parmaceutical, MSD, Sysmex, Ortho Clinical Diagnostics, and Daiichi Sankyo.
Figures

Similar articles
-
Vitamin K Antagonist Reversal for Urgent Surgery Using 4-Factor Prothrombin Complex Concentrates: A Randomized Clinical Trial.JAMA Netw Open. 2024 Aug 1;7(8):e2424758. doi: 10.1001/jamanetworkopen.2024.24758. JAMA Netw Open. 2024. PMID: 39088218 Free PMC article. Clinical Trial.
-
Hemostatic Efficacy and Safety of Weight-Based Versus Fixed-Dose 4F-PCC for Vitamin K Antagonist Reversal.J Pharm Pract. 2024 Oct;37(5):1099-1106. doi: 10.1177/08971900241228779. Epub 2024 Jan 19. J Pharm Pract. 2024. PMID: 38241183
-
Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial.Lancet. 2015 May 23;385(9982):2077-87. doi: 10.1016/S0140-6736(14)61685-8. Epub 2015 Feb 27. Lancet. 2015. PMID: 25728933 Free PMC article. Clinical Trial.
-
Fixed-Dose Four-Factor Prothrombin Complex Concentrate for Vitamin K Antagonist Reversal: Does One Dose Fit All?Pharmacotherapy. 2019 May;39(5):599-608. doi: 10.1002/phar.2261. Epub 2019 Apr 21. Pharmacotherapy. 2019. PMID: 30892733 Review.
-
Roles of Four-Factor Prothrombin Complex Concentrate in the Management of Critical Bleeding.Transfus Med Rev. 2021 Oct;35(4):96-103. doi: 10.1016/j.tmrv.2021.06.007. Epub 2021 Aug 26. Transfus Med Rev. 2021. PMID: 34551881 Review.
Cited by
-
Efficacy and Safety of Prothrombin complex Concentrate in Patients with Massive Intraoperative Bleeding During non-Cardiac Surgery: A Retrospective Cohort Study.Clin Appl Thromb Hemost. 2025 Jan-Dec;31:10760296251356202. doi: 10.1177/10760296251356202. Epub 2025 Jul 2. Clin Appl Thromb Hemost. 2025. PMID: 40605362 Free PMC article.
-
Real-World Safety and Effectiveness of a 4-Factor Prothrombin Complex Concentrate in Japanese Patients Experiencing Major Bleeding: A Post-marketing Surveillance Study.Cardiol Ther. 2024 Mar;13(1):221-232. doi: 10.1007/s40119-024-00357-6. Epub 2024 Feb 6. Cardiol Ther. 2024. PMID: 38319533 Free PMC article.
-
JCS/JHRS 2020 Guideline on Pharmacotherapy of Cardiac Arrhythmias.J Arrhythm. 2022 Oct 25;38(6):833-973. doi: 10.1002/joa3.12714. eCollection 2022 Dec. J Arrhythm. 2022. PMID: 36524037 Free PMC article. No abstract available.
-
Safety and Effectiveness of Four-Factor Prothrombin Complex Concentrate in Special Populations with INR Below 2: A Post-Marketing Surveillance Study.Cardiol Ther. 2024 Sep;13(3):603-614. doi: 10.1007/s40119-024-00380-7. Epub 2024 Aug 3. Cardiol Ther. 2024. PMID: 39096439 Free PMC article.
References
-
- Keeling D, Baglin T, Tait C, et al. . Guidelines on oral anticoagulation with warfarin - fourth edition. Br J Haematol. 2011;154(3):311-324. - PubMed
-
- Nishimura RA, Otto CM, Bonow RO, et al. . 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(25):e1159-e1195. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

