Direct β- and γ-C(sp3 )-H Alkynylation of Free Carboxylic Acids*
- PMID: 32898310
- PMCID: PMC7756274
- DOI: 10.1002/anie.202010784
Direct β- and γ-C(sp3 )-H Alkynylation of Free Carboxylic Acids*
Abstract
In this study we report the identification of a novel class of ligands for palladium-catalyzed C(sp3 )-H activation that enables the direct alkynylation of free carboxylic acid substrates. In contrast to previous synthetic methods, no introduction/removal of an exogenous directing group is required. A broad scope of acids including both α-quaternary and challenging α-non-quaternary can be used as substrates. Additionally, the alkynylation in the distal γ-position is reported. Finally, this study encompasses preliminary findings on an enantioselective variant of the title transformation as well as synthetic applications of the products obtained.
Keywords: Alkynylation; Carboxylic acids; C−H activation; Ligand-enabled catalysis; Palladium.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
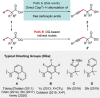
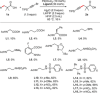
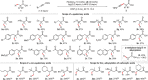
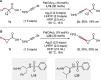


References
-
- None
-
- Modern Acetylene Chemistry, Wiley-VCH, Weinheim, 1995;
-
- Larock R. C., Comprehensive Organic Transformations: A Guide to Functional Group Preparations, Wiley-VCH, Weinheim, 1999;
-
- Gorzynski Smith J., Org. Chem. McGraw-Hill Education, 2008;
-
- Lutz J. F., Zarafshani Z., Adv. Drug Delivery Rev. 2008, 60, 958. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

