Transfer of metastatic traits via miR-200c in extracellular vesicles derived from colorectal cancer stem cells is inhibited by atractylenolide I
- PMID: 32898324
- PMCID: PMC7423185
- DOI: 10.1002/ctm2.139
Transfer of metastatic traits via miR-200c in extracellular vesicles derived from colorectal cancer stem cells is inhibited by atractylenolide I
Abstract
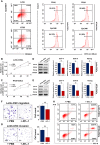
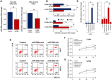
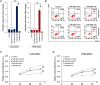
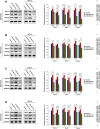
Cancer stem cells (CSCs) are important factors contributing to tumorigenesis. We examined whether CSCs isolated from colorectal cancer (CRC) cells possess metastatic properties that can be transferred to non-CSCs via the delivery of miR-200c enclosed in extracellular vesicles (EVs). The inhibitory effect of atractylenolide I (ATL-1), a traditional Chinese medicinal compound, on miR-200c activity and metastatic transfer was investigated. EVs were isolated from colorectal CSCs. The expression of miR-200c was evaluated in CSCs and CSC-derived EVs, and horizontal transfer of metastatic properties via EVs to non-CSCs was investigated in terms of cell behavior and phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling. CSCs isolated from metastatic CRC cells exhibited higher levels of miR-200c than those in nonmetastatic CRC cells. Overexpression of miR-200c in CSCs enhanced metastatic potential by promoting proliferation and inhibiting apoptosis, in turn leading to the release of EVs carrying an excess of miR-200c. Non-CSCs co-cultured with miR-200c-containing EVs exhibited enhanced invasion and stemness maintenance associated with PI3K/Akt/mTOR activation, demonstrating successful metastatic transfer via EV delivery. Furthermore, ATL-1 impaired the EV-mediated transfer of metastatic properties by suppressing miR-200c activity and disrupting EV uptake by non-CSCs. EVs are critical signal transducers that facilitate intercellular communication and exchange of metastatic properties, which can be controlled by ATL-1. The findings are useful in the development of microRNA-based anticancer strategies by targeting EV-mediated activity, especially using natural compounds.
Keywords: PI3K/Akt/mTOR; atractylenolide I; extracellular vesicles; metastasis; stemness.
© 2020 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Atractylenolide I inhibits colorectal cancer cell proliferation by affecting metabolism and stemness via AKT/mTOR signaling.Phytomedicine. 2020 Mar;68:153191. doi: 10.1016/j.phymed.2020.153191. Epub 2020 Feb 14. Phytomedicine. 2020. PMID: 32135457
-
Ovatodiolide Suppresses Oral Cancer Malignancy by Down-Regulating Exosomal Mir-21/STAT3/β-Catenin Cargo and Preventing Oncogenic Transformation of Normal Gingival Fibroblasts.Cancers (Basel). 2019 Dec 24;12(1):56. doi: 10.3390/cancers12010056. Cancers (Basel). 2019. PMID: 31878245 Free PMC article.
-
Overexpression of the miR-141/200c cluster promotes the migratory and invasive ability of triple-negative breast cancer cells through the activation of the FAK and PI3K/AKT signaling pathways by secreting VEGF-A.BMC Cancer. 2016 Aug 2;16:570. doi: 10.1186/s12885-016-2620-7. BMC Cancer. 2016. PMID: 27484639 Free PMC article.
-
Regulation of colorectal carcinoma stemness, growth, and metastasis by an miR-200c-Sox2-negative feedback loop mechanism.Clin Cancer Res. 2014 May 15;20(10):2631-42. doi: 10.1158/1078-0432.CCR-13-2348. Epub 2014 Mar 21. Clin Cancer Res. 2014. PMID: 24658157
-
Extracellular Vesicle- and Mitochondria-Based Targeting of Non-Small Cell Lung Cancer Response to Radiation: Challenges and Perspectives.Cancers (Basel). 2024 Jun 15;16(12):2235. doi: 10.3390/cancers16122235. Cancers (Basel). 2024. PMID: 38927940 Free PMC article. Review.
Cited by
-
Extracellular vesicles and cancer stemness in hepatocellular carcinoma - is there a link?Front Immunol. 2024 Feb 27;15:1368898. doi: 10.3389/fimmu.2024.1368898. eCollection 2024. Front Immunol. 2024. PMID: 38476233 Free PMC article. Review.
-
Transcriptional regulation of cancer stem cell: regulatory factors elucidation and cancer treatment strategies.J Exp Clin Cancer Res. 2024 Apr 2;43(1):99. doi: 10.1186/s13046-024-03021-y. J Exp Clin Cancer Res. 2024. PMID: 38561775 Free PMC article. Review.
-
Extracellular vesicles package dsDNA to aggravate Crohn's disease by activating the STING pathway.Cell Death Dis. 2021 Aug 27;12(9):815. doi: 10.1038/s41419-021-04101-z. Cell Death Dis. 2021. PMID: 34453041 Free PMC article.
-
Modulatory effects of cancer stem cell-derived extracellular vesicles on the tumor immune microenvironment.Front Immunol. 2024 Jun 19;15:1362120. doi: 10.3389/fimmu.2024.1362120. eCollection 2024. Front Immunol. 2024. PMID: 38962016 Free PMC article. Review.
-
Chemical Constitution, Pharmacological Effects and the Underlying Mechanism of Atractylenolides: A Review.Molecules. 2023 May 9;28(10):3987. doi: 10.3390/molecules28103987. Molecules. 2023. PMID: 37241729 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
