COVID-19 diagnostic testing: Technology perspective
- PMID: 32898340
- PMCID: PMC7443140
- DOI: 10.1002/ctm2.158
COVID-19 diagnostic testing: Technology perspective
Abstract
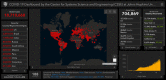
The corona virus disease 2019 (COVID-19) is a highly contagious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). More than 18 million people were infected with a total of 0.7 million deaths in ∼188 countries. Controlling the spread of SARS-CoV-2 is therefore inherently dependent on identifying and isolating infected individuals, especially since COVID-19 can result in little to no symptoms. Here, we provide a comprehensive review of the different primary technologies used to test for COVID-19 infection, discuss the advantages and disadvantages of each technology, and highlight the studies that have employed them. We also describe technologies that have the potential to accelerate SARS-CoV-2 detection in the future, including digital PCR, CRISPR, and microarray. Finally, remaining challenges in COVID-19 diagnostic testing are discussed, including (a) the lack of universal standards for diagnostic testing; (b) the identification of appropriate sample collection site(s); (c) the difficulty in performing large population screening; and (d) the limited understanding of SARS-COV-2 viral invasion, replication, and transmission.
Keywords: COVID-19; SARS-COV-2; diagnostic test; nucleic acid; serum.
© 2020 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
M. X., D. W., H. W., X. Z., T. L., J. D., M. L., J. Z., and K. Z., searched the literature. M. X., D. X., and X. Y. prepared the manuscript.
The authors have declared no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
