The root cause of drug resistance in HER2-positive breast cancer and the therapeutic approaches to overcoming the resistance
- PMID: 32898548
- PMCID: PMC7855784
- DOI: 10.1016/j.pharmthera.2020.107677
The root cause of drug resistance in HER2-positive breast cancer and the therapeutic approaches to overcoming the resistance
Abstract
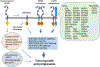
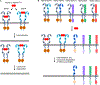
HER2 is a well-known oncogenic receptor tyrosine kinase. HER2 gene amplification occurs in about 20% of breast cancer (BC), which leads to overexpression of HER2 protein, known as HER2-positive BC. Inhibitors of HER2 have significantly improved the prognosis of patients with this subset of BC. Since 1998, seven HER2 inhibitors have been developed to treat this disease. However, drug resistance is common and remains a major unresolved clinical problem. Patients typically show disease progression after some time on treatment. This review discusses the complexity and diversified nature of HER2 signaling, the mechanisms of actions and therapeutic activities of all HER2 inhibitors, the roles of HER2 and other signaling proteins in HER2-positive BC resistant to the inhibitors, the non-cell-autonomous mechanisms of drug resistance, and the heterogeneity of tumor HER2 expression. The review presents the concept that drug resistance in HER2-positive BC results primarily from the inability of HER2 inhibitors to deplete HER2. Emerging therapeutics that are promising for overcoming drug resistance are also discussed.
Keywords: Cancer treatment; HER2 inhibitor; PEPD(G278D); Receptor tyrosine kinase; Targeted therapy; Therapeutic resistance.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The author declares that there are no conflicts of interest.
Figures
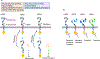
References
-
- Andre F, O’Regan R, Ozguroglu M, Toi M, Xu B, Jerusalem G, … Gianni L (2014). Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet Oncology 15, 580–591. - PubMed
-
- Arpino G, Gutierrez C, Weiss H, Rimawi M, Massarweh S, Bharwani L, … Schiff R (2007). Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. Journal of the National Cancer Institute 99, 694–705. - PubMed
-
- Arribas J, Baselga J, Pedersen K, & Parra-Palau JL (2011). p95HER2 and breast cancer. Cancer Research 71, 1515–1519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

