Analysis of type I IFN response and T cell activation in severe COVID-19/HIV-1 coinfection: A case report
- PMID: 32899009
- PMCID: PMC7478511
- DOI: 10.1097/MD.0000000000021803
Analysis of type I IFN response and T cell activation in severe COVID-19/HIV-1 coinfection: A case report
Erratum in
-
Analysis of type I IFN response and T cell activation in severe COVID-19/HIV-1 coinfection: A case report: Erratum.Medicine (Baltimore). 2020 Oct 16;99(42):e22949. doi: 10.1097/MD.0000000000022949. Medicine (Baltimore). 2020. PMID: 33080768 Free PMC article. No abstract available.
Abstract
Rationale: Complex immune dysregulation in interferon (IFN) and T cell response has been observed in human immunodeficiency virus (HIV-1)-infected patients as well as in coronavirus disease-2019 (COVID-19) patients. However, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)/HIV-1 coinfection has been described in only few cases worldwide and no data are available on immunological outcomes in HIV-1-patients infected with SARS-CoV-2. Hence, this study aims to compare type I IFN response and T cell activation levels between a SARS-CoV-2/HIV-1-coinfected female patient and age-matched HIV-1-positive or uninfected women.
Patient concerns: A 52-year-old woman diagnosed with SARS-CoV-2/HIV-1 coinfection, ten HIV-1-positive women and five age-matched-healthy individuals were enrolled in this study.
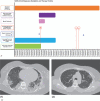
Diagnoses: SARS-CoV-2 infection caused severe pneumonia in the second week of illness in HIV-1-positive patient under protease inhibitors. Chest high-resolution computed tomography images of the SARS-CoV-2/HIV-1-coinfected patient showed bilateral ground-glass opacities.
Interventions: SARS-CoV-2/HIV-1-coinfected female patient under darunavir/cobicistat regimen received a 7-days hydroxychloroquine therapy. Analysis of IFNα/β mRNA levels and CD4 and CD8 T cell (CD38, human leukocyte antigen-DR [HLA-DR], CD38 HLA-DR) frequencies were performed by RT/real-time PCR assays and flow cytometry, respectively. Median relative difference (MRD) was calculated for each immunological variable. For values greater than reference, MRD should be a positive number and for values that are smaller, MRD should be negative.
Outcomes: The severe pneumonia observed in SARS-CoV-2/HIV-1-positive patient under protease inhibitors was reversed by a 7-days hydroxychloroquine therapy. At the end of treatment, on day 7, patient reported resolution of fever, normalization of respiratory rate (14 breaths/min), and improved oxygen arterial pressure with a FiO2 of 30%. MRD values for IFNα/β and CD4 and CD8 T cells expressing CD38 and/or HLA-DR found in SARS-CoV-2-/HIV-1-coinfected woman were approximatively equal to 0 when refereed respectively to HIV-1-positive female patients [MRDs IFNα/β: median -0.2545 (range: -0.5/0.1); T cells: median -0.11 (range: -0.8/1.3)] and ≥ 6 when referred to healthy individuals [MRDs IFNα/β: median 28.45 (range: 15/41.9); T cells: median 10 (range 6/22)].
Lessons: These results indicate that SARS-CoV-2 infection in HIV-1-positive female patient was associated with increased levels of IFNα/β-mRNAs and T cell activation compared to healthy individuals.
Conflict of interest statement
The authors have no conflicts of interests to disclose.
Figures

Similar articles
-
Novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) co-infection with HIV: clinical case series analysis in North Central Nigeria.Pan Afr Med J. 2020 Sep 10;37:47. doi: 10.11604/pamj.2020.37.47.24200. eCollection 2020. Pan Afr Med J. 2020. PMID: 33209174 Free PMC article.
-
Effect of a Previous History of Antiretroviral Treatment on Clinical Picture of Patients with Co-infection of SARS-CoV-2 and HIV: A Preliminary Study.Int J Infect Dis. 2020 Nov;100:141-148. doi: 10.1016/j.ijid.2020.08.045. Epub 2020 Aug 20. Int J Infect Dis. 2020. PMID: 32829051 Free PMC article.
-
Case report: one case of coronavirus disease 2019 (COVID-19) in a patient co-infected by HIV with a normal CD4+ T cell count.AIDS Res Ther. 2020 Jul 23;17(1):46. doi: 10.1186/s12981-020-00301-3. AIDS Res Ther. 2020. PMID: 32703286 Free PMC article.
-
Coronavirus disease 2019 (COVID-19) in a patient with ankylosing spondylitis treated with secukinumab: a case-based review.Rheumatol Int. 2020 Oct;40(10):1707-1716. doi: 10.1007/s00296-020-04635-z. Epub 2020 Jun 26. Rheumatol Int. 2020. PMID: 32591970 Free PMC article. Review.
-
High levels of anti-SSA/Ro antibodies in COVID-19 patients with severe respiratory failure: a case-based review : High levels of anti-SSA/Ro antibodies in COVID-19.Clin Rheumatol. 2020 Nov;39(11):3171-3175. doi: 10.1007/s10067-020-05359-y. Epub 2020 Aug 25. Clin Rheumatol. 2020. PMID: 32844364 Free PMC article. Review.
Cited by
-
Analysis of type I IFN response and T cell activation in severe COVID-19/HIV-1 coinfection: A case report: Erratum.Medicine (Baltimore). 2020 Oct 16;99(42):e22949. doi: 10.1097/MD.0000000000022949. Medicine (Baltimore). 2020. PMID: 33080768 Free PMC article. No abstract available.
-
Clinical outcome of patients with HIV/SARS-COV-2 co-infection.Med J Armed Forces India. 2022 Nov 18;80(5):604-6. doi: 10.1016/j.mjafi.2022.09.003. Online ahead of print. Med J Armed Forces India. 2022. PMID: 36415858 Free PMC article. No abstract available.
-
COVID-19 in People Living with HIV: A Systematic Review and Meta-Analysis.Int J Environ Res Public Health. 2021 Mar 30;18(7):3554. doi: 10.3390/ijerph18073554. Int J Environ Res Public Health. 2021. PMID: 33808066 Free PMC article.
-
Oral Bacteriotherapy Reduces the Occurrence of Chronic Fatigue in COVID-19 Patients.Front Nutr. 2022 Jan 12;8:756177. doi: 10.3389/fnut.2021.756177. eCollection 2021. Front Nutr. 2022. PMID: 35096923 Free PMC article.
-
Distribution of Interferon Lambda 4 Single Nucleotide Polymorphism rs11322783 Genotypes in Patients with COVID-19.Microorganisms. 2022 Feb 4;10(2):363. doi: 10.3390/microorganisms10020363. Microorganisms. 2022. PMID: 35208821 Free PMC article.
References
-
- World Health Organization Coronavirus disease 2019 (COVID-19) Situation Report. Published online, April 2020.
-
- González Álvarez DA, López Cortés LF, Cordero E. Impact of HIV on the severity of influenza. Expert Rev Respir Med 2016;10:463–72.. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

