Efficacy and safety of thread embedding acupuncture on knee osteoarthritis: A randomized, controlled, pilot clinical trial
- PMID: 32899030
- PMCID: PMC7478827
- DOI: 10.1097/MD.0000000000021957
Efficacy and safety of thread embedding acupuncture on knee osteoarthritis: A randomized, controlled, pilot clinical trial
Abstract
Introduction: Although there are various therapeutic methods for the treatment of knee osteoarthritis, each has its advantages and shortcomings, and a definitive treatment method is yet to be determined. This pilot study is designed to obtain basic data for a further large-scale trial as well as provide information about the feasibility of thread embedding acupuncture (TEA) with polydioxanone thread in knee osteoarthritis patients.
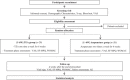
Methods and analysis: This study is a clinical trial to evaluate the efficacy and safety of TEA for knee osteoarthritis. Forty participants will be recruited at the hospital and after randomization into 2 groups of 20 (experimental and control); they will be treated for 6 weeks. The experimental group will receive TEA treatment 6 times (1 time/week) in 6 weeks on 14 defined knee areas, and the control group, acupuncture treatments 12 times (2 times/week) in 6 weeks on 9 defined acupuncture points. The visual analogue scale (VAS) will be used for the primary efficacy assessment and Short-form McGill Pain Questionnaire (SF-MPQ), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) will be used for the secondary efficacy assessment. The follow-ups before clinical trial, 3 weeks after procedure, 6 weeks after procedure, and 4 weeks after the end of procedure will be done to compare the degree of pain with the control group, which received the acupuncture treatment.
Conclusion: The trial based on this study will provide clinical information on the efficacy and safety of TEA treatment on knee osteoarthritis.
Trial registration number: KCT0004844.
Conflict of interest statement
None of the authors declares any conflicts of interest.
Figures
References
-
- Gerwin N, Hops C, Lucke A. Intraarticular drug delivery in osteoarthritis. Adv Drug Delivery Rev 2006;58:226–42.. - PubMed
-
- Bliddal H, Christensen R. The treatment and prevention of knee osteoarthritis: a tool for clinical decision-making. Expert Opin Pharmacother 2009;10:1793–804.. - PubMed
-
- Ondrésik M, Azevedo Maia FR, da Silva Morais A, et al. Management of knee osteoarthritis. Current status and future trends. Biotechnol Bioeng 2017;114:717–39.. - PubMed
-
- Kon E, Filardo G, Drobnic M, et al. Non-surgical management of early knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2012;20:436–9.. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical