Manipulation of JAK/STAT Signalling by High-Risk HPVs: Potential Therapeutic Targets for HPV-Associated Malignancies
- PMID: 32899142
- PMCID: PMC7552066
- DOI: 10.3390/v12090977
Manipulation of JAK/STAT Signalling by High-Risk HPVs: Potential Therapeutic Targets for HPV-Associated Malignancies
Abstract
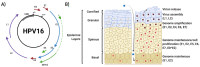
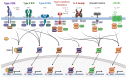
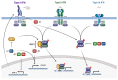
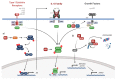
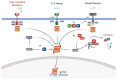
Human papillomaviruses (HPVs) are small, DNA viruses that cause around 5% of all cancers in humans, including almost all cervical cancer cases and a significant proportion of anogenital and oral cancers. The HPV oncoproteins E5, E6 and E7 manipulate cellular signalling pathways to evade the immune response and promote virus persistence. The Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT) pathway has emerged as a key mediator in a wide range of important biological signalling pathways, including cell proliferation, cell survival and the immune response. While STAT1 and STAT2 primarily drive immune signalling initiated by interferons, STAT3 and STAT5 have widely been linked to the survival and proliferative potential of a number of cancers. As such, the inhibition of STAT3 and STAT5 may offer a therapeutic benefit in HPV-associated cancers. In this review, we will discuss how HPV manipulates JAK/STAT signalling to evade the immune system and promote cell proliferation, enabling viral persistence and driving cancer development. We also discuss approaches to inhibit the JAK/STAT pathway and how these could potentially be used in the treatment of HPV-associated disease.
Keywords: HPV; JAK; STAT; cancer; cytokine signalling; interferon signalling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

