Cellular and Subcellular Localisation of Kv4-Associated KChIP Proteins in the Rat Cerebellum
- PMID: 32899153
- PMCID: PMC7503578
- DOI: 10.3390/ijms21176403
Cellular and Subcellular Localisation of Kv4-Associated KChIP Proteins in the Rat Cerebellum
Abstract
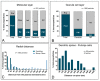
The K+ channel interacting proteins (KChIPs) are a family of cytosolic proteins that interact with Kv4 channels, leading to higher current density, modulation of channel inactivation and faster recovery from inactivation. Using immunohistochemical techniques at the light and electron microscopic level combined with quantitative analysis, we investigated the cellular and subcellular localisation of KChIP3 and KChIP4 to compare their distribution patterns with those for Kv4.2 and Kv4.3 in the cerebellar cortex. Immunohistochemistry at the light microscopic level demonstrated that KChIP3, KChIP4, Kv4.2 and Kv4.3 proteins were widely expressed in the cerebellum, with mostly overlapping patterns. Immunoelectron microscopic techniques showed that KChIP3, KChIP4, Kv4.2 and Kv4.3 shared virtually the same somato-dendritic domains of Purkinje cells and granule cells. Application of quantitative approaches showed that KChIP3 and KChIP4 were mainly membrane-associated, but also present at cytoplasmic sites close to the plasma membrane, in dendritic spines and shafts of Purkinje cells (PCs) and dendrites of granule cells (GCs). Similarly, immunoparticles for Kv4.2 and Kv4.3 were observed along the plasma membrane and at intracellular sites in the same neuron populations. In addition to the preferential postsynaptic distribution, KChIPs and Kv4 were also distributed presynaptically in parallel fibres and mossy fibres. Immunoparticles for KChIP3, KChIP4 and Kv4.3 were detected in parallel fibres, and KChIP3, KChIP4, Kv4.2 and Kv4.3 were found in parallel fibres, indicating that composition of KChIP and Kv4 seems to be input-dependent. Together, our findings unravelled previously uncharacterised KChIP and Kv4 subcellular localisation patterns in neurons, revealed that KChIP have additional Kv4-unrelated functions in the cerebellum and support the formation of macromolecular complexes between KChIP3 and KChIP4 with heterotetrameric Kv4.2/Kv4.3 channels.
Keywords: KChIP proteins; cerebellum; electron microscopy; immunohistochemistry; potassium channel.
Conflict of interest statement
The authors of this manuscript declare that they have no competing interest.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

