Do Your Kids Get What You Paid for? Evaluation of Commercially Available Probiotic Products Intended for Children in the Republic of the Philippines and the Republic of Korea
- PMID: 32899215
- PMCID: PMC7555838
- DOI: 10.3390/foods9091229
Do Your Kids Get What You Paid for? Evaluation of Commercially Available Probiotic Products Intended for Children in the Republic of the Philippines and the Republic of Korea
Abstract
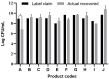
A wide range of probiotic products is available on the market and can be easily purchased over the counter and unlike pharmaceutical drugs, their commercial distribution is not strictly regulated. In this study, ten probiotic preparations commercially available for children's consumption in the Republic of the Philippines (PH) and the Republic of Korea (SK) have been investigated. The analyses included determination of viable counts and taxonomic identification of the bacterial species present in each formulation. The status of each product was assessed by comparing the results with information and claims provided on the label. In addition to their molecular identification, safety assessment of the isolated strains was conducted by testing for hemolysis, biogenic amine production and antibiotic resistance. One out of the ten products contained lower viable numbers of recovered microorganisms than claimed on the label. Enterococcus strains, although not mentioned on the label, were isolated from four products. Some of these isolates produced biogenic amines and were resistant to one or several antibiotics. Metagenomic analyses of two products revealed that one product did not contain most of the microorganisms declared in its specification. The study demonstrated that some commercial probiotic products for children did not match their label claims. Infants and young children belong to the most vulnerable members of society, and food supplements including probiotics destined for this consumer group require careful checking and strict regulation before commercial distribution.
Keywords: children; label claims; lactic acid bacteria; molecular identification; probiotics; safety; viable count.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. This study was strictly for academic and informative purposes and not intended as quality control for any agency or company.
Figures
Similar articles
-
Microbiological Testing of Probiotic Preparations.Int J Environ Res Public Health. 2022 May 7;19(9):5701. doi: 10.3390/ijerph19095701. Int J Environ Res Public Health. 2022. PMID: 35565098 Free PMC article. Review.
-
Evaluation of Probiotic Characteristics of Lactic Acid Bacteria Isolated from Two Commercial Preparations Available in Indian Market.Indian J Microbiol. 2019 Mar;59(1):112-115. doi: 10.1007/s12088-018-0762-9. Epub 2018 Sep 26. Indian J Microbiol. 2019. PMID: 30728640 Free PMC article.
-
Bioinformatics-Based Tools and Software in Clinical Research: A New Emerging Area.Methods Mol Biol. 2019;1939:215-230. doi: 10.1007/978-1-4939-9089-4_12. Methods Mol Biol. 2019. PMID: 30848464
-
Market and product assessment of probiotic/prebiotic-containing functional foods and supplements manufactured in South Africa.S Afr Med J. 2005 Feb;95(2):114-9. S Afr Med J. 2005. PMID: 15751206
-
[Probiotics--possibilities and limitations of their application in food, animal feed, and in pharmaceutical preparations for men and animals].Berl Munch Tierarztl Wochenschr. 2001 Nov-Dec;114(11-12):410-9. Berl Munch Tierarztl Wochenschr. 2001. PMID: 11766268 Review. German.
Cited by
-
Assessing the authenticity and purity of a commercial Bacillus thuringiensis bioinsecticide through whole genome sequencing and metagenomics approaches.Front Microbiol. 2025 Jan 31;16:1532788. doi: 10.3389/fmicb.2025.1532788. eCollection 2025. Front Microbiol. 2025. PMID: 39963497 Free PMC article.
-
Microbiological Testing of Probiotic Preparations.Int J Environ Res Public Health. 2022 May 7;19(9):5701. doi: 10.3390/ijerph19095701. Int J Environ Res Public Health. 2022. PMID: 35565098 Free PMC article. Review.
-
Microbiological Quality and Resistance to an Artificial Gut Environment of Two Probiotic Formulations.Foods. 2021 Nov 12;10(11):2781. doi: 10.3390/foods10112781. Foods. 2021. PMID: 34829062 Free PMC article.
-
Role of Probiotics in the Treatment and Prevention of Common Gastrointestinal Conditions in Children.Pediatr Gastroenterol Hepatol Nutr. 2024 Jan;27(1):1-14. doi: 10.5223/pghn.2024.27.1.1. Epub 2024 Jan 9. Pediatr Gastroenterol Hepatol Nutr. 2024. PMID: 38249642 Free PMC article. Review.
-
Evaluation of Dietary Supplements Containing Viable Bacteria by Cultivation/MALDI-TOF Mass Spectrometry and PCR Identification.Front Microbiol. 2021 Jul 19;12:700138. doi: 10.3389/fmicb.2021.700138. eCollection 2021. Front Microbiol. 2021. PMID: 34349743 Free PMC article.
References
-
- Schneider C., Illi M., Lötscher M., Wehrli M., Von Gunten S. Isolation of Antibodies from Human Plasma, Saliva, Breast Milk, and Gastrointestinal Fluid. In: Kaveri S.V., Bayry J., editors. Natural Antibodies. Volume 1643. Humana Press; New York, NY, USA: 2017. pp. 23–31. (Methods in Molecular Biology). - PubMed
-
- Canani R.B., Cirillo P., Terrin G., Cesarano L., Spagnuolo M.I., Vincenzo A.D., Albano F., Passariello A., Marco G.D., Manguso F., et al. Probiotics for treatment of acute diarrhoea in children: Randomised clinical trial of five different preparations. BMJ. 2007;335:340. doi: 10.1136/bmj.39272.581736.55. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases