Antibiotics Differentially Modulate Lipoteichoic Acid-Mediated Host Immune Response
- PMID: 32899240
- PMCID: PMC7558621
- DOI: 10.3390/antibiotics9090573
Antibiotics Differentially Modulate Lipoteichoic Acid-Mediated Host Immune Response
Abstract
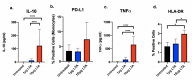
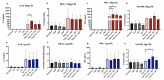
In Staphylococcus aureus bacteremia, our group has shown that a dysregulated balance of pro- and anti-inflammatory cytokine response biased towards an immunoparalysis phenotype is predictive of persistence and mortality, despite receipt of antibiotics. Certain antibiotics, as well as lipoteichoic acid (LTA) released from S. aureus, can modulate immune response ex vivo. Here, we evaluated the effects of three anti-staphylococcal antibiotics (vancomycin, tedizolid, and daptomycin) on the expression of cytokines and cell surface markers of immune activation (TNFα, HLA-DR) and immunoparalysis (IL-10, PD-L1) in human peripheral blood mononuclear cells (PBMC) exposed to high (10 μg) and low (1 μg) doses of LTA. Results suggested a dose-dependent relationship between LTA and induction of anti- and pro-inflammatory immune responses. Differential antibiotic effects were prominently observed at high but not low LTA condition. Vancomycin significantly induced IL-10 and TNFα expression, whereas daptomycin had no effects on cytokine response or expression of cell surface receptors. Tedizolid increased TNFα and modestly increased HLA-DR expression, suggesting a stimulatory effect. These findings suggest that anti-staphylococcal agents differentially alter LTA-mediated immune cell activation status and cytokine response, providing support for future clinical studies to better elucidate the complexities of host-microbial-antibiotic interaction that can help direct precision therapy for S. aureus bacteremia.
Keywords: Staphylococcus aureus bacteremia; antibiotics; immunomodulation; lipoteichoic acid; sepsis immunoparalysis.
Conflict of interest statement
Cubist, now Merck, which provided partial funding for this study to Annie Wong-Beringer., had no role in study design, data collection and interpretation, or the decision to submit the work for publication. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. Marquerita Algorri has no conflicts of interest to declare.
Figures
References
-
- Minejima E., Bensman J., She R.C., Mack W.J., Tran M.T., Ny P., Lou M., Yamaki J., Nieberg P., Ho J., et al. A Dysregulated Balance of Proinflammatory and Anti-Inflammatory Host Cytokine Response Early During Therapy Predicts Persistence and Mortality in Staphylococcus aureus Bacteremia. Crit. Care Med. 2016;44:671–679. doi: 10.1097/CCM.0000000000001465. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

