Mast Cells, microRNAs and Others: The Role of Translational Research on Colorectal Cancer in the Forthcoming Era of Precision Medicine
- PMID: 32899322
- PMCID: PMC7564551
- DOI: 10.3390/jcm9092852
Mast Cells, microRNAs and Others: The Role of Translational Research on Colorectal Cancer in the Forthcoming Era of Precision Medicine
Abstract
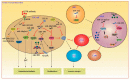
Colorectal cancer (CRC) is a heterogeneous disease, molecularly and anatomically, that develops in a multi-step process requiring the accumulation of several genetic or epigenetic mutations that lead to the gradual transformation of normal mucosa into cancer. In fact, tumorigenesis is extremely complex, with many immunologic and non-immunologic factors present in the tumor microenvironment that can influence tumorigenesis. In the last few years, a role for mast cells (MCs), microRNAs (miRNAs), Kirsten rat sarcoma (KRAS) and v-raf murine sarcoma viral oncogene homologue B (BRAF) in cancer development and progression has been suggested, and numerous efforts have been made to thoroughly assess their correlation with CRC to improve patient survival and quality of life. The identification of easily measurable, non-invasive and cost-effective biomarkers, the so-called "ideal biomarkers", for CRC screening and treatment remains a high priority. The aim of this review is to discuss the emerging role of mast cells (MCs), microRNAs (miRNAs), KRAS and BRAF as diagnostic and prognostic biomarkers for CRC, evaluating their influence as potential therapy targets in the forthcoming era of precision medicine.
Keywords: BRAF; KRAS; colorectal cancer; mast cells; microRNAs; precision medicine; translational research.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Alvaro E., Cano J.M., Garcia J.L., Brandariz L., Olmedillas-Lopez S., Arriba M., Rueda D., Rodriguez Y., Canete A., Arribas J., et al. Clinical and Molecular Comparative Study of Colorectal Cancer Based on Age-of-Onset and Tumor Location: Two Main Criteria for Subclassifying Colorectal Cancer. Int. J. Mol. Sci. 2019;20:968. doi: 10.3390/ijms20040968. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

