Sensitivity, Specificity and Predictive Values of Molecular and Serological Tests for COVID-19: A Longitudinal Study in Emergency Room
- PMID: 32899333
- PMCID: PMC7555224
- DOI: 10.3390/diagnostics10090669
Sensitivity, Specificity and Predictive Values of Molecular and Serological Tests for COVID-19: A Longitudinal Study in Emergency Room
Abstract
Background: We assessed the sensitivity, specificity and positive and negative predictive value (PPV and NPV) of molecular and serological tests for the diagnosis of SARS-CoV-2 infection.
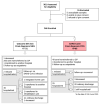
Methods: A total of 346 patients were enrolled in the emergency room. We evaluated three Reverse Transcriptase-real time PCRs (RT-PCRs) including six different gene targets, five serologic rapid diagnostic tests (RDT) and one ELISA. The final classification of infected/non-infected patients was performed using Latent Class Analysis combined with clinical re-assessment of incongruous cases.
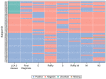
Results: Out of these, 24.6% of patients were classified as infected. The molecular test RQ-SARS-nCoV-2 showed the highest performance with 91.8% sensitivity, 100% specificity, 100.0% PPV and 97.4% NPV respectively. Considering the single gene targets, S and RdRp of RQ-SARS-nCoV-2 had the highest sensitivity (94.1%). The in-house RdRp presented the lowest sensitivity (62.4%). The specificity ranged from 99.2% for in-house RdRp and N2 to 95.0% for E. The PPV ranged from 97.1% of N2 to 85.4% of E and the NPV from 98.1% of S to 89.0% of in-house RdRp. All serological tests had < 50% sensitivity and low PPV and NPV. VivaDiag IgM (RDT) had 98.5% specificity, with 84.0% PPV, but 24.7% sensitivity.
Conclusion: Molecular tests for SARS-CoV-2 infection showed excellent specificity, but significant differences in sensitivity. Serological tests have limited utility in a clinical context.
Keywords: RT-PCR; SARS-CoV-2; accuracy; diagnosis; serology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- WHO Coronavirus Disease (COVID-19) Dashboard. [(accessed on 27 August 2020)]; Available online: https://covid19.who.int/
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

