Attenuated Influenza Virions Expressing the SARS-CoV-2 Receptor-Binding Domain Induce Neutralizing Antibodies in Mice
- PMID: 32899480
- PMCID: PMC7552029
- DOI: 10.3390/v12090987
Attenuated Influenza Virions Expressing the SARS-CoV-2 Receptor-Binding Domain Induce Neutralizing Antibodies in Mice
Abstract
An effective vaccine is essential for controlling the spread of the SARS-CoV-2 virus. Here, we describe an influenza virus-based vaccine for SARS-CoV-2. We incorporated a membrane-anchored form of the SARS-CoV-2 spike receptor binding domain (RBD) in place of the neuraminidase (NA) coding sequence in an influenza virus also possessing a mutation that reduces the affinity of hemagglutinin for its sialic acid receptor. The resulting ΔNA(RBD)-Flu virus can be generated by reverse genetics and grown to high titers in cell culture. A single-dose intranasal inoculation of mice with ΔNA(RBD)-Flu elicits serum neutralizing antibody titers against SAR-CoV-2 comparable to those observed in humans following natural infection (~1:200). Furthermore, ΔNA(RBD)-Flu itself causes no apparent disease in mice. It might be possible to produce a vaccine similar to ΔNA(RBD)-Flu at scale by leveraging existing platforms for the production of influenza vaccines.
Keywords: RBD; SARS-CoV-2; influenza; intranasal; live attenuated vaccine; spike.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
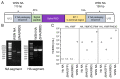

Update of
-
Attenuated influenza virions expressing the SARS-CoV-2 receptor-binding domain induce neutralizing antibodies in mice.bioRxiv [Preprint]. 2020 Sep 3:2020.08.12.248823. doi: 10.1101/2020.08.12.248823. bioRxiv. 2020. Update in: Viruses. 2020 Sep 05;12(9):E987. doi: 10.3390/v12090987. PMID: 32817935 Free PMC article. Updated. Preprint.
Comment on
-
Pregnancy and fertility-related adverse outcomes associated with Chlamydia trachomatis infection: a global systematic review and meta-analysis.Sex Transm Infect. 2020 Aug;96(5):322-329. doi: 10.1136/sextrans-2019-053999. Epub 2019 Dec 13. Sex Transm Infect. 2020. PMID: 31836678 Free PMC article.
References
-
- World Health Organization . Coronavirus disease (COVID-19) Situation Report-183. World Health Organization; Geneva, Switzerland: 2020.
-
- Meisner J., Szretter K.J., Bradley K.C., Langley W.A., Li Z.-N., Lee B.-J., Thoennes S., Martin J., Skehel J.J., Russell R.J., et al. Infectivity Studies of Influenza Virus Hemagglutinin Receptor Binding Site Mutants in Mice. J. Virol. 2008;82:5079–5083. doi: 10.1128/JVI.01958-07. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01AI141707, R01AI127893, F30AI149928/National Institute of Allergy and Infectious Diseases/International
- R01 AI127893/AI/NIAID NIH HHS/United States
- T32 GM007266/GM/NIGMS NIH HHS/United States
- R01 AI141707/AI/NIAID NIH HHS/United States
- Investigator Award/HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

