Neuroprotective Effect of Piracetam against Cocaine-Induced Neuro Epigenetic Modification of DNA Methylation in Astrocytes
- PMID: 32899583
- PMCID: PMC7565945
- DOI: 10.3390/brainsci10090611
Neuroprotective Effect of Piracetam against Cocaine-Induced Neuro Epigenetic Modification of DNA Methylation in Astrocytes
Abstract
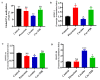
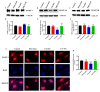
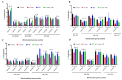
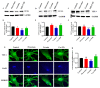
Cocaine abuse is known to alter mitochondrial biogenesis and induce epigenetic modification linked with neuronal dysfunction. Cocaine-induced epigenetic modification of DNA methylation and the mitochondrial genome may affect mitochondrial DNA (mtDNA) and nuclear DNA (nDNA), as epigenetic DNA methylation is key to maintaining genomic integrity in the central nervous system (CNS). However, the impact of cocaine-mediated epigenetic changes in astrocytes has not yet been elucidated. In this study, we explored the neuroprotective effect of piracetam against cocaine-induced epigenetic changes in DNA methylation in astrocytes. To study our hypothesis, we exposed human astrocytes to cocaine alone or in combination with the nootropic drug piracetam. We examined the expression of the DNA methyltransferases (DNMTs) DNMT-1, DNMT-3A, and DNMT-3B; global DNA methylation levels of 5-methycytosine (5-mC); and induction of ten-eleven translocation (TET) enzymes in astrocytes. In addition, we analyzed mtDNA methylation by targeted next-generation bisulfite sequencing. Our data provide evidence that cocaine impairs DNMT activity and thereby has impacts on mtDNA, which might contribute to the neurodegeneration observed in cocaine users. These effects might be at least partially prevented by piracetam, allowing neuronal function to be maintained.
Keywords: DNMTs; TET and astrocytes; cocaine; piracetam.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Influence of psychostimulants and opioids on epigenetic modification of class III histone deacetylase (HDAC)-sirtuins in glial cells.Sci Rep. 2021 Oct 29;11(1):21335. doi: 10.1038/s41598-021-00836-z. Sci Rep. 2021. PMID: 34716387 Free PMC article.
-
Psychostimulants and opioids differentially influence the epigenetic modification of histone acetyltransferase and histone deacetylase in astrocytes.PLoS One. 2021 Jun 11;16(6):e0252895. doi: 10.1371/journal.pone.0252895. eCollection 2021. PLoS One. 2021. PMID: 34115777 Free PMC article.
-
HIV-1 Tat and cocaine impact mitochondrial epigenetics: effects on DNA methylation.Epigenetics. 2021 Sep;16(9):980-999. doi: 10.1080/15592294.2020.1834919. Epub 2020 Oct 24. Epigenetics. 2021. PMID: 33100130 Free PMC article.
-
Local chromatin microenvironment determines DNMT activity: from DNA methyltransferase to DNA demethylase or DNA dehydroxymethylase.Epigenetics. 2015;10(8):671-6. doi: 10.1080/15592294.2015.1062204. Epigenetics. 2015. PMID: 26098813 Free PMC article. Review.
-
Mitochondrial DNA methylation as a next-generation biomarker and diagnostic tool.Mol Genet Metab. 2013 Sep-Oct;110(1-2):25-34. doi: 10.1016/j.ymgme.2013.07.012. Epub 2013 Jul 19. Mol Genet Metab. 2013. PMID: 23920043 Review.
Cited by
-
Influence of psychostimulants and opioids on epigenetic modification of class III histone deacetylase (HDAC)-sirtuins in glial cells.Sci Rep. 2021 Oct 29;11(1):21335. doi: 10.1038/s41598-021-00836-z. Sci Rep. 2021. PMID: 34716387 Free PMC article.
-
Solving the Global Opioid Crisis: Incorporating Genetic Addiction Risk Assessment with Personalized Dopaminergic Homeostatic Therapy and Awareness Integration Therapy.J Addict Psychiatry. 2024;8(1):50-95. Epub 2024 Jun 20. J Addict Psychiatry. 2024. PMID: 39635461 Free PMC article.
-
Psychostimulants and opioids differentially influence the epigenetic modification of histone acetyltransferase and histone deacetylase in astrocytes.PLoS One. 2021 Jun 11;16(6):e0252895. doi: 10.1371/journal.pone.0252895. eCollection 2021. PLoS One. 2021. PMID: 34115777 Free PMC article.
-
Substance-Induced Psychiatric Disorders, Epigenetic and Microbiome Alterations, and Potential for Therapeutic Interventions.Brain Sci. 2024 Jul 30;14(8):769. doi: 10.3390/brainsci14080769. Brain Sci. 2024. PMID: 39199463 Free PMC article. Review.
-
HIV-1 Tat and cocaine impact astrocytic energy reservoirs and epigenetic regulation by influencing the LINC01133-hsa-miR-4726-5p-NDUFA9 axis.Mol Ther Nucleic Acids. 2022 Jul 6;29:243-258. doi: 10.1016/j.omtn.2022.07.001. eCollection 2022 Sep 13. Mol Ther Nucleic Acids. 2022. PMID: 35892093 Free PMC article.
References
-
- United Nations Office on Drugs and Crime. [(accessed on 27 July 2020)]; Available online: https://www.unodc.org/
Grants and funding
LinkOut - more resources
Full Text Sources

