A Higher Proportion of Eicosapentaenoic Acid (EPA) When Combined with Docosahexaenoic Acid (DHA) in Omega-3 Dietary Supplements Provides Higher Antioxidant Effects in Human Retinal Cells
- PMID: 32899655
- PMCID: PMC7555332
- DOI: 10.3390/antiox9090828
A Higher Proportion of Eicosapentaenoic Acid (EPA) When Combined with Docosahexaenoic Acid (DHA) in Omega-3 Dietary Supplements Provides Higher Antioxidant Effects in Human Retinal Cells
Abstract
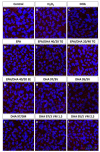
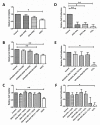
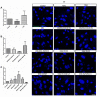
Retinal pigment epithelium (RPE) is a key regulator of retinal function and is directly related to the transport, delivery, and metabolism of long-chain n-3 polyunsaturated fatty acids (n3-PUFA), in the retina. Due to their functions and location, RPE cells are constantly exposed to oxidative stress. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have shown to have antioxidant effects by different mechanisms. For this reason, we designed an in vitro study to compare 10 formulations of DHA and EPA supplements from different origins and combined in different proportions, evaluating their effect on cell viability, cell proliferation, reactive oxygen species production, and cell migration using ARPE-19 cells. Furthermore, we assessed their ability to rescue RPE cells from the oxidative conditions seen in diabetic retinopathy. Our results showed that the different formulations of n3-PUFAs have a beneficial effect on cell viability and proliferation and are able to restore oxidative induced RPE damage. We observed that the n3-PUFA provided different results alone or combined in the same supplement. When combined, the best results were obtained in formulations that included a higher proportion of EPA than DHA. Moreover, n3-PUFA in the form of ethyl-esters had a worse performance when compared with triglycerides or phospholipid based formulations.
Keywords: diabetic retinopathy; docosahexaenoic acid (DHA); eicosapentaenoic acid (EPA); oxidative stress; retinal pigment epithelium.
Conflict of interest statement
AGL is a consultant for Bayer, Novartis, Allergan, Thea, and Roche. The rest of the authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

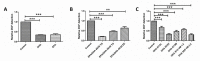


Similar articles
-
Similar eicosapentaenoic acid and docosahexaenoic acid plasma levels achieved with fish oil or krill oil in a randomized double-blind four-week bioavailability study.Lipids Health Dis. 2015 Sep 2;14:99. doi: 10.1186/s12944-015-0109-z. Lipids Health Dis. 2015. PMID: 26328782 Free PMC article. Clinical Trial.
-
Dietary Docosahexaenoic Acid (DHA) and Eicosapentaenoic Acid (EPA) Operate by Different Mechanisms to Modulate Hepatic Steatosis and Hyperinsulemia in fa/fa Zucker Rats.Nutrients. 2019 Apr 24;11(4):917. doi: 10.3390/nu11040917. Nutrients. 2019. PMID: 31022865 Free PMC article.
-
Effect of dietary EPA and DHA on murine blood and liver fatty acid profile and liver oxylipin pattern depending on high and low dietary n6-PUFA.Food Funct. 2020 Oct 21;11(10):9177-9191. doi: 10.1039/d0fo01462a. Food Funct. 2020. PMID: 33030169
-
EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials.J Am Coll Nutr. 2009 Oct;28(5):525-42. doi: 10.1080/07315724.2009.10719785. J Am Coll Nutr. 2009. PMID: 20439549 Review.
-
Aspirin and omega-3 polyunsaturated fatty acid use and their interaction in cardiovascular diseases and colorectal adenomas.Nutr Res Rev. 2022 Dec;35(2):295-307. doi: 10.1017/S0954422421000238. Epub 2021 Jul 13. Nutr Res Rev. 2022. PMID: 34253265 Review.
Cited by
-
Antioxidant Activity and Neuroprotective Role of Docosahexaenoic Acid (DHA) Supplementation in Eye Diseases That Can Lead to Blindness: A Narrative Review.Antioxidants (Basel). 2021 Mar 5;10(3):386. doi: 10.3390/antiox10030386. Antioxidants (Basel). 2021. PMID: 33807538 Free PMC article. Review.
-
Recent Developments in Diabetic Retinal Neurodegeneration: A Literature Review.J Diabetes Res. 2020 Dec 7;2020:5728674. doi: 10.1155/2020/5728674. eCollection 2020. J Diabetes Res. 2020. PMID: 34151902 Free PMC article. Review.
-
Mitochondrial Dysfunction and Endoplasmic Reticulum Stress in Age Related Macular Degeneration, Role in Pathophysiology, and Possible New Therapeutic Strategies.Antioxidants (Basel). 2021 Jul 23;10(8):1170. doi: 10.3390/antiox10081170. Antioxidants (Basel). 2021. PMID: 34439418 Free PMC article. Review.
-
Oxidative Stress in Diabetic Retinopathy.Antioxidants (Basel). 2021 Jan 4;10(1):50. doi: 10.3390/antiox10010050. Antioxidants (Basel). 2021. PMID: 33406579 Free PMC article.
-
Chinmedomics Strategy for Elucidating the Pharmacological Effects and Discovering Bioactive Compounds From Keluoxin Against Diabetic Retinopathy.Front Pharmacol. 2022 Mar 31;13:728256. doi: 10.3389/fphar.2022.728256. eCollection 2022. Front Pharmacol. 2022. PMID: 35431942 Free PMC article.
References
-
- Flaxman S.R., Bourne R.R.A., Resnikoff S., Ackland P., Braithwaite T., Cicinelli M.V., Das A., Jonas J.B., Keeffe J., Kempen J., et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Heal. 2017;5 doi: 10.1016/S2214-109X(17)30393-5. - DOI - PubMed
-
- Elisa D-NET—Connecting Diabetes Professionals Wordwide. [(accessed on 29 July 2020)]; Available online: https://d-net.idf.org.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

