Dance with the Devil: Stress Granules and Signaling in Antiviral Responses
- PMID: 32899736
- PMCID: PMC7552005
- DOI: 10.3390/v12090984
Dance with the Devil: Stress Granules and Signaling in Antiviral Responses
Abstract
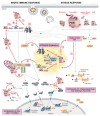
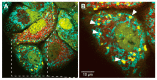
Cells have evolved highly specialized sentinels that detect viral infection and elicit an antiviral response. Among these, the stress-sensing protein kinase R, which is activated by double-stranded RNA, mediates suppression of the host translation machinery as a strategy to limit viral replication. Non-translating mRNAs rapidly condensate by phase separation into cytosolic stress granules, together with numerous RNA-binding proteins and components of signal transduction pathways. Growing evidence suggests that the integrated stress response, and stress granules in particular, contribute to antiviral defense. This review summarizes the current understanding of how stress and innate immune signaling act in concert to mount an effective response against virus infection, with a particular focus on the potential role of stress granules in the coordination of antiviral signaling cascades.
Keywords: G3BP1; PKR; antiviral signaling; innate immune response; stress granules; stress response; virus.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Colson P., De Lamballerie X., Yutin N., Asgari S., Bigot Y., Bideshi D.K., Cheng X.W., Federici B.A., Van Etten J.L., Koonin E.V., et al. “Megavirales”, a proposed new order for eukaryotic nucleocytoplasmic large DNA viruses. Arch. Virol. 2013;158:2517–2521. doi: 10.1007/s00705-013-1768-6. - DOI - PMC - PubMed
-
- Sullivan M.B., Huang K.H., Ignacio-Espinoza J.C., Berlin A.M., Kelly L., Weigele P.R., DeFrancesco A.S., Kern S.E., Thompson L.R., Young S., et al. Genomic analysis of oceanic cyanobacterial myoviruses compared with T4-like myoviruses from diverse hosts and environments. Environ. Microbiol. 2010;12:3035–3056. doi: 10.1111/j.1462-2920.2010.02280.x. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

