The Potential of Algal Biotechnology to Produce Antiviral Compounds and Biopharmaceuticals
- PMID: 32899754
- PMCID: PMC7571207
- DOI: 10.3390/molecules25184049
The Potential of Algal Biotechnology to Produce Antiviral Compounds and Biopharmaceuticals
Abstract
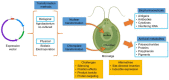
The emergence of the Coronavirus Disease 2019 (COVID-19) caused by the SARS-CoV-2 virus has led to an unprecedented pandemic, which demands urgent development of antiviral drugs and antibodies; as well as prophylactic approaches, namely vaccines. Algae biotechnology has much to offer in this scenario given the diversity of such organisms, which are a valuable source of antiviral and anti-inflammatory compounds that can also be used to produce vaccines and antibodies. Antivirals with possible activity against SARS-CoV-2 are summarized, based on previously reported activity against Coronaviruses or other enveloped or respiratory viruses. Moreover, the potential of algae-derived anti-inflammatory compounds to treat severe cases of COVID-19 is contemplated. The scenario of producing biopharmaceuticals in recombinant algae is presented and the cases of algae-made vaccines targeting viral diseases is highlighted as valuable references for the development of anti-SARS-CoV-2 vaccines. Successful cases in the production of functional antibodies are described. Perspectives on how specific algae species and genetic engineering techniques can be applied for the production of anti-viral compounds antibodies and vaccines against SARS-CoV-2 are provided.
Keywords: COVID-19; Chlamydomonas reinhardtii; MERS-CoV; SARS-CoV-2; monoclonal antibody; recombinant antigen; transplastomic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- World Health Organization Coronavirus Disease 2019 (COVID-19) Situation Report—51, 2020. [(accessed on 11 March 2020)]; Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

