Natural Products Repertoire of the Red Sea
- PMID: 32899763
- PMCID: PMC7551641
- DOI: 10.3390/md18090457
Natural Products Repertoire of the Red Sea
Abstract
Marine natural products have achieved great success as an important source of new lead compounds for drug discovery. The Red Sea provides enormous diversity on the biological scale in all domains of life including micro- and macro-organisms. In this review, which covers the literature to the end of 2019, we summarize the diversity of bioactive secondary metabolites derived from Red Sea micro- and macro-organisms, and discuss their biological potential whenever applicable. Moreover, the diversity of the Red Sea organisms is highlighted as well as their genomic potential. This review is a comprehensive study that compares the natural products recovered from the Red Sea in terms of ecological role and pharmacological activities.
Keywords: Red Sea; bioactivity; biodiversity; marine metagenomics; marine natural products; marine organisms.
Conflict of interest statement
The authors declare there is no conflict of interest.
Figures




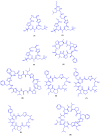
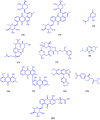

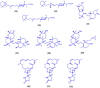
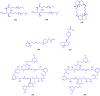













References
-
- Bosworth W., Huchon P., McClay K. The red sea and gulf of aden basins. J. Afr. Earth Sci. 2005;43:334–378. doi: 10.1016/j.jafrearsci.2005.07.020. - DOI
-
- Berumen M.L., Hoey A.S., Bass W.H., Bouwmeester J., Catania D., Cochran J.E.M., Khalil M.T., Miyake S., Mughal M.R., Spaet J.L.Y., et al. The status of coral reef ecology research in the red sea. Coral Reefs. 2013;32:737–748. doi: 10.1007/s00338-013-1055-8. - DOI
-
- DiBattista J.D., Roberts M.B., Bouwmeester J., Bowen B.W., Coker D.J., Lozano-Cortés D.F., Howard Choat J., Gaither M.R., Hobbs J.-P.A., Khalil M.T., et al. A review of contemporary patterns of endemism for shallow water reef fauna in the red sea. J. Biogeogr. 2016;43:423–439. doi: 10.1111/jbi.12649. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

