Biomarkers for Alzheimer's Disease Early Diagnosis
- PMID: 32899797
- PMCID: PMC7563965
- DOI: 10.3390/jpm10030114
Biomarkers for Alzheimer's Disease Early Diagnosis
Abstract
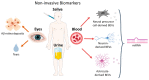
Alzheimer's disease (AD) is the most common cause of dementia, affecting the central nervous system (CNS) through the accumulation of intraneuronal neurofibrillary tau tangles (NFTs) and β-amyloid plaques. By the time AD is clinically diagnosed, neuronal loss has already occurred in many brain and retinal regions. Therefore, the availability of early and reliable diagnosis markers of the disease would allow its detection and taking preventive measures to avoid neuronal loss. Current diagnostic tools in the brain, such as magnetic resonance imaging (MRI), positron emission tomography (PET) imaging, and cerebrospinal fluid (CSF) biomarkers (Aβ and tau) detection are invasive and expensive. Brain-secreted extracellular vesicles (BEVs) isolated from peripheral blood have emerged as novel strategies in the study of AD, with enormous potential as a diagnostic evaluation of therapeutics and treatment tools. In addition; similar mechanisms of neurodegeneration have been demonstrated in the brain and the eyes of AD patients. Since the eyes are more accessible than the brain, several eye tests that detect cellular and vascular changes in the retina have also been proposed as potential screening biomarkers. The aim of this study is to summarize and discuss several potential markers in the brain, eye, blood, and other accessible biofluids like saliva and urine, and correlate them with earlier diagnosis and prognosis to identify individuals with mild symptoms prior to dementia.
Keywords: Alzheimer’s disease; biofluids; biomarkers; early diagnosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease.J Neurovirol. 2019 Oct;25(5):702-709. doi: 10.1007/s13365-018-0695-4. Epub 2019 Jan 4. J Neurovirol. 2019. PMID: 30610738 Free PMC article. Review.
-
Amyloid Beta Immunoreactivity in the Retinal Ganglion Cell Layer of the Alzheimer's Eye.Front Neurosci. 2020 Jul 31;14:758. doi: 10.3389/fnins.2020.00758. eCollection 2020. Front Neurosci. 2020. PMID: 32848548 Free PMC article.
-
Non-Invasive Retinal Biomarkers for Early Diagnosis of Alzheimer's Disease.Biomedicines. 2025 Jan 24;13(2):283. doi: 10.3390/biomedicines13020283. Biomedicines. 2025. PMID: 40002697 Free PMC article. Review.
-
Current Biomarkers for Alzheimer's Disease: From CSF to Blood.J Pers Med. 2020 Aug 12;10(3):85. doi: 10.3390/jpm10030085. J Pers Med. 2020. PMID: 32806668 Free PMC article. Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
Effects of Donepezil Treatment on Brain Metabolites, Gut Microbiota, and Gut Metabolites in an Amyloid Beta-Induced Cognitive Impairment Mouse Pilot Model.Molecules. 2022 Oct 5;27(19):6591. doi: 10.3390/molecules27196591. Molecules. 2022. PMID: 36235127 Free PMC article.
-
Editorial: Saliva used as biological fluid to detect neurodegenerative and neurodevelopmental diseases.Front Neurosci. 2023 Feb 28;17:1141376. doi: 10.3389/fnins.2023.1141376. eCollection 2023. Front Neurosci. 2023. PMID: 36925736 Free PMC article. No abstract available.
-
PET imaging of retinal inflammation in mice exposed to blue light using [18F]-DPA-714.Mol Vis. 2023 Jul 16;29:117-124. eCollection 2023. Mol Vis. 2023. PMID: 37859807 Free PMC article.
-
Technological advances in electrochemical biosensors for the detection of disease biomarkers.Biomed Eng Lett. 2021 Aug 27;11(4):309-334. doi: 10.1007/s13534-021-00204-w. eCollection 2021 Nov. Biomed Eng Lett. 2021. PMID: 34466275 Free PMC article. Review.
-
Advanced Overview of Biomarkers and Techniques for Early Diagnosis of Alzheimer's Disease.Cell Mol Neurobiol. 2023 Aug;43(6):2491-2523. doi: 10.1007/s10571-023-01330-y. Epub 2023 Feb 27. Cell Mol Neurobiol. 2023. PMID: 36847930 Free PMC article. Review.
References
-
- Dubois B., Feldman H.H., Jacova C., DeKosky S.T., Barberger-Gateau P., Cummings J., Delacourte A., Galasko D., Gauthier S., Jicha G., et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. - DOI - PubMed
-
- La Joie R., Perrotin A., De La Sayette V., Egret S., Doeuvre L., Belliard S., Eustache F., Desgranges B., Chételat G. Hippocampal subfield volumetry in mild cognitive impairment, Alzheimer’s disease and semantic dementia. NeuroImage Clin. 2013;3:155–162. doi: 10.1016/j.nicl.2013.08.007. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

