Tolerance of Stored Boar Spermatozoa to Autologous Seminal Plasma: A Proteomic and Lipidomic Approach
- PMID: 32899843
- PMCID: PMC7555833
- DOI: 10.3390/ijms21186474
Tolerance of Stored Boar Spermatozoa to Autologous Seminal Plasma: A Proteomic and Lipidomic Approach
Abstract
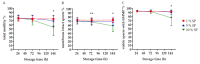
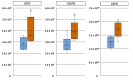
Long-term exposure of liquid preserved boar spermatozoa to seminal plasma (SP) can cause dramatic sperm injury. This study examined whether boar specificity exists in the sensitivity of spermatozoa to SP and whether correspondent biomarkers can be identified. Consecutive ejaculates (n = 4-5) collected from 19 boars were centrifuged, diluted with a pH-stablising extender with 10% (v/v) autologous SP and evaluated by computer-assisted semen analysis and flow cytometry. Up until 144 h storage, four boars showed consistently high sperm motility, viability and mitochondria activity, and one boar showed consistently low values. Intra-boar variability was high in the other boars. Screening of SP (n = 12 samples) for protein markers using mass spectrometry identified three protein candidates of which the granulin precursor, legumain and AWN were 0.5 to 0.9 log2-fold less abundant (p < 0.05) in SP-resistant compared to SP-sensitive samples. Lipidome analysis by mass spectrometry revealed 568 lipids showing no difference between the SP-groups. The most abundant lipids were cholesterol (42,442 pmol), followed by phosphatidylserine (20,956 pmol) and ether-linked phosphatidylethanolamine (13,039 pmol). In conclusion, three candidate proteins were identified which might be indicative of SP-tolerance of sperm during long-term storage. Noteworthy, a first lipidomic profile of boar SP is presented.
Keywords: biomarker; boar seminal plasma; lipidomics; proteomics; semen preservation; sperm quality.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The authors declare no conflict of interest.
Figures






References
-
- Pavaneli A.P.P., Passarelli M.D.S., De Freitas F.V., Ravagnani G.M., Torres M.A., Martins S.M.M.K., Yeste M., Andrade A. Removal of seminal plasma prior to liquid storage of boar spermatozoa: A practice that can improve their fertilizing ability. Theriogenology. 2019;125:79–86. doi: 10.1016/j.theriogenology.2018.10.020. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

