P2 × 7 Receptor Inhibits Astroglial Autophagy via Regulating FAK- and PHLPP1/2-Mediated AKT-S473 Phosphorylation Following Kainic Acid-Induced Seizures
- PMID: 32899862
- PMCID: PMC7555659
- DOI: 10.3390/ijms21186476
P2 × 7 Receptor Inhibits Astroglial Autophagy via Regulating FAK- and PHLPP1/2-Mediated AKT-S473 Phosphorylation Following Kainic Acid-Induced Seizures
Abstract
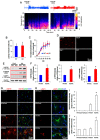
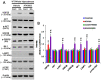
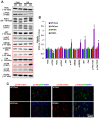
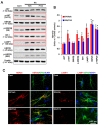
Recently, we have reported that blockade/deletion of P2X7 receptor (P2X7R), an ATP-gated ion channel, exacerbates heat shock protein 25 (HSP25)-mediated astroglial autophagy (clasmatodendrosis) following kainic acid (KA) injection. In P2X7R knockout (KO) mice, prolonged astroglial HSP25 induction exerts 5' adenosine monophosphate-activated protein kinase/unc-51 like autophagy activating kinase 1-mediated autophagic pathway independent of mammalian target of rapamycin (mTOR) activity following KA injection. Sustained HSP25 expression also enhances AKT-serine (S) 473 phosphorylation leading to astroglial autophagy via glycogen synthase kinase-3β/bax interacting factor 1 signaling pathway. However, it is unanswered how P2X7R deletion induces AKT-S473 hyperphosphorylation during autophagic process in astrocytes. In the present study, we found that AKT-S473 phosphorylation was increased by enhancing activity of focal adhesion kinase (FAK), independent of mTOR complex (mTORC) 1 and 2 activities in isolated astrocytes of P2X7R knockout (KO) mice following KA injection. In addition, HSP25 overexpression in P2X7R KO mice acted as a chaperone of AKT, which retained AKT-S473 phosphorylation by inhibiting the pleckstrin homology domain and leucine-rich repeat protein phosphatase (PHLPP) 1- and 2-binding to AKT. Therefore, our findings suggest that P2X7R may be a fine-tuner of AKT-S473 activity during astroglial autophagy by regulating FAK phosphorylation and HSP25-mediated inhibition of PHLPP1/2-AKT binding following KA treatment.
Keywords: Bif-1; FAK inhibitor 14; LAMP1; PRAS40; Raptor; Rictor; p70S6K; siRNA.
Conflict of interest statement
The authors have declared that no conflict of interest exist.
Figures






References
-
- Penfield W. Neuroglia and microglia—The interstitial tissue of the central nervous system. In: Cowdry E.V., editor. Special Cytology, the Form and Function of the Cell in Health and Disease. Hoeber; New York, NY, USA: 1928. pp. 1033–1068.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

