Tetrel Bonding and Other Non-Covalent Interactions Assisted Supramolecular Aggregation in a New Pb(II) Complex of an Isonicotinohydrazide
- PMID: 32899863
- PMCID: PMC7571010
- DOI: 10.3390/molecules25184056
Tetrel Bonding and Other Non-Covalent Interactions Assisted Supramolecular Aggregation in a New Pb(II) Complex of an Isonicotinohydrazide
Abstract
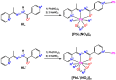
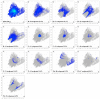
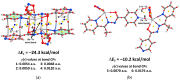
A new supramolecular Pb(II) complex [PbL(NO2)]n was synthesized from Pb(NO3)2, N'-(1-(pyridin-2-yl)ethylidene)isonicotinohydrazide (HL) and NaNO2. [PbL(NO2)]n is constructed from discrete [PbL(NO2)] units with an almost ideal N2O3 square pyramidal coordination environment around Pb(II). The ligand L- is coordinated through the 2-pyridyl N-atom, one aza N-atom, and the carbonyl O-atom. The nitrite ligand binds in a κ2-O,O coordination mode through both O-atoms. The Pb(II) center exhibits a hemidirected coordination geometry with a pronounced coordination gap, which allows a close approach of two additional N-atoms arising from the N=C(O) N-atom of an adjacent molecule and from the 4-pyridyl N-atom from the another adjacent molecule, yielding a N4O3 coordination, constructed from two Pb-N and three Pb-O covalent bonds, and two Pb⋯N tetrel bonds. Dimeric units in the structure of [PbL(NO2)]n are formed by the Pb⋯N=C(O) tetrel bonds and intermolecular electrostatically enforced π+⋯π- stacking interactions between the 2- and 4-pyridyl rings and further stabilized by C-H⋯π intermolecular interactions, formed by one of the methyl H-atoms and the 4-pyridyl ring. These dimers are embedded in a 2D network representing a simplified uninodal 3-connected fes (Shubnikov plane net) topology defined by the point symbol (4∙82). The Hirshfeld surface analysis of [PbL(NO2)] revealed that the intermolecular H⋯X (X = H, C, N, O) contacts occupy an overwhelming majority of the molecular surface of the [PbL(NO2)] coordination unit. Furthermore, the structure is characterized by intermolecular C⋯C and C⋯N interactions, corresponding to the intermolecular π⋯π stacking interactions. Notably, intermolecular Pb⋯N and, most interestingly, Pb⋯H interactions are remarkable contributors to the molecular surface of [PbL(NO2)]. While the former contacts are due to the Pb⋯N tetrel bonds, the latter contacts are mainly due to the interaction with the methyl H-atoms in the π⋯π stacked [PbL(NO2)] molecules. Molecular electrostatic potential (MEP) surface calculations showed marked electrostatic contributions to both the Pb⋯N tetrel bonds and the dimer forming π+⋯π- stacking interactions. Quantum theory of atoms in molecules (QTAIM) analyses underlined the tetrel bonding character of the Pb⋯N interactions. The manifold non-covalent interactions found in this supramolecular assembly are the result of the proper combination of the polyfunctional multidentate pyridine-hydrazide ligand and the small nitrito auxiliary ligand.
Keywords: DFT calculations; Hirshfeld surface analysis; crystal structure; isonicotinohydrazide; lead(II); non-covalent interaction; tetrel bond.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
The tetrel bonding role in supramolecular aggregation of lead(II) acetate and a thiosemicarbazide derivative.Acta Crystallogr B Struct Sci Cryst Eng Mater. 2022 Aug 1;78(Pt 4):685-694. doi: 10.1107/S2052520622005789. Epub 2022 Jul 22. Acta Crystallogr B Struct Sci Cryst Eng Mater. 2022. PMID: 35975834
-
Synthesis, Crystal Structures, Hirshfeld Surface Analysis, Computational Investigations, Thermal Properties, and Electrochemical Analysis of Two New Cu(II) and Co(II) Coordination Polymers with the Ligand 5-Methyl-1-(pyridine-4-yl-methyl)-1H-1,2,3-triazole-4-carboxylate.Int J Mol Sci. 2025 Feb 15;26(4):1671. doi: 10.3390/ijms26041671. Int J Mol Sci. 2025. PMID: 40004136 Free PMC article.
-
Synthesis, spectroscopic findings and crystal engineering of Pb(ii)-Salen coordination polymers, and supramolecular architectures engineered by σ-hole/spodium/tetrel bonds: a combined experimental and theoretical investigation.RSC Adv. 2022 Feb 24;12(10):6352-6363. doi: 10.1039/d1ra09346k. eCollection 2022 Feb 16. RSC Adv. 2022. PMID: 35424552 Free PMC article.
-
A Supramolecular Approach to Structure-Based Design with A Focus on Synthons Hierarchy in Ornithine-Derived Ligands: Review, Synthesis, Experimental and in Silico Studies.Molecules. 2020 Mar 3;25(5):1135. doi: 10.3390/molecules25051135. Molecules. 2020. PMID: 32138329 Free PMC article. Review.
-
The Relevance of Experimental Charge Density Analysis in Unraveling Noncovalent Interactions in Molecular Crystals.Molecules. 2022 Jun 8;27(12):3690. doi: 10.3390/molecules27123690. Molecules. 2022. PMID: 35744821 Free PMC article. Review.
Cited by
-
A Nanosized Porous Supramolecular Lead(II)-N'-phenyl(pyridin-2-yl)methylene-N-phenylthiosemicarbazide Aggregate, Obtained Under Electrochemical Conditions.Inorg Chem. 2024 Oct 7;63(40):18581-18588. doi: 10.1021/acs.inorgchem.4c02182. Epub 2024 Sep 26. Inorg Chem. 2024. PMID: 39324362 Free PMC article.
-
Anion-Anion Interactions in Aerogen-Bonded Complexes. Influence of Solvent Environment.Molecules. 2021 Apr 7;26(8):2116. doi: 10.3390/molecules26082116. Molecules. 2021. PMID: 33917030 Free PMC article.
-
Interaction of Lead(II) Perchlorate with N'-Isonicotinoylpicolinohydrazonamide and Its Sodium Salt in the Presence of Potassium Cyanide: Yellow Green Light Emitting Phosphors, Stabilized by Tetrel Bonds, and a System to Transform Methanol to Acetate.Inorg Chem. 2025 Jul 21;64(28):14670-14683. doi: 10.1021/acs.inorgchem.5c02419. Epub 2025 Jul 9. Inorg Chem. 2025. PMID: 40631779 Free PMC article.
-
Static and Dynamical Quantum Studies of CX3-AlX2 and CSiX3-BX2 (X = F, Cl, Br) Complexes with Hydrocyanic Acid: Unusual Behavior of Strong π-Hole at Triel Center.Int J Mol Sci. 2023 Apr 26;24(9):7881. doi: 10.3390/ijms24097881. Int J Mol Sci. 2023. PMID: 37175586 Free PMC article.
References
-
- Van der Waals J.D. Ph.D. Thesis. University of Leiden; Leiden, The Netherlands: 1873. Over de Continuiteit van den Gas-en Vloiestoftoestand.
-
- Scheiner S. New ideas from old concepts: The hydrogen bond. Biochemist. 2019;41:6–9. doi: 10.1042/BIO04104006. - DOI
-
- Hobza P., Zahradník R. Intermolecular Interactions between Medium-Sized Systems. Nonempirical and Empirical Calculations of Interaction Energies: Successes and Failures. Chem. Rev. 1988;88:871–897. doi: 10.1021/cr00088a004. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

