Gateways for Glutamate Neuroprotection in Parkinson's Disease (PD): Essential Role of EAAT3 and NCX1 Revealed in an In Vitro Model of PD
- PMID: 32899900
- PMCID: PMC7563499
- DOI: 10.3390/cells9092037
Gateways for Glutamate Neuroprotection in Parkinson's Disease (PD): Essential Role of EAAT3 and NCX1 Revealed in an In Vitro Model of PD
Abstract
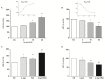
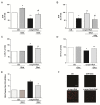
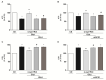
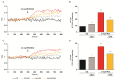
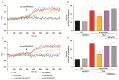
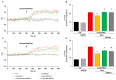
Increasing evidence suggests that metabolic alterations may be etiologically linked to neurodegenerative disorders such as Parkinson's disease (PD) and in particular empathizes the possibility of targeting mitochondrial dysfunctions to improve PD progression. Under different pathological conditions (i.e., cardiac and neuronal ischemia/reperfusion injury), we showed that supplementation of energetic substrates like glutamate exerts a protective role by preserving mitochondrial functions and enhancing ATP synthesis through a mechanism involving the Na+-dependent excitatory amino acid transporters (EAATs) and the Na+/Ca2+ exchanger (NCX). In this study, we investigated whether a similar approach aimed at promoting glutamate metabolism would be also beneficial against cell damage in an in vitro PD-like model. In retinoic acid (RA)-differentiated SH-SY5Y cells challenged with α-synuclein (α-syn) plus rotenone (Rot), glutamate significantly improved cell viability by increasing ATP levels, reducing oxidative damage and cytosolic and mitochondrial Ca2+ overload. Glutamate benefits were strikingly lost when either EAAT3 or NCX1 expression was knocked down by RNA silencing. Overall, our results open the possibility of targeting EAAT3/NCX1 functions to limit PD pathology by simultaneously favoring glutamate uptake and metabolic use in dopaminergic neurons.
Keywords: EAAT3; NCX1; Parkinson’s disease; glutamate; mitochondrial dysfunction; neuronal survival.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A non-redundant role of EAAT3 for ATP synthesis mediated by GDH in dopaminergic neuronal cells: a new avenue for glutamate metabolism and protection in Parkinson's disease.FEBS J. 2025 Jun;292(12):3224-3241. doi: 10.1111/febs.70053. Epub 2025 Mar 5. FEBS J. 2025. PMID: 40045666 Free PMC article.
-
Glutamate as a potential "survival factor" in an in vitro model of neuronal hypoxia/reoxygenation injury: leading role of the Na+/Ca2+ exchanger.Cell Death Dis. 2018 Jun 28;9(7):731. doi: 10.1038/s41419-018-0784-6. Cell Death Dis. 2018. PMID: 29955038 Free PMC article.
-
NCX1 and EAAC1 transporters are involved in the protective action of glutamate in an in vitro Alzheimer's disease-like model.Cell Calcium. 2020 Nov;91:102268. doi: 10.1016/j.ceca.2020.102268. Epub 2020 Aug 11. Cell Calcium. 2020. PMID: 32827867
-
NCX and EAAT transporters in ischemia: At the crossroad between glutamate metabolism and cell survival.Cell Calcium. 2020 Mar;86:102160. doi: 10.1016/j.ceca.2020.102160. Epub 2020 Jan 13. Cell Calcium. 2020. PMID: 31962228 Review.
-
Glutamate transport and metabolism in dopaminergic neurons of substantia nigra: implications for the pathogenesis of Parkinson's disease.J Neurol. 2000 Apr;247 Suppl 2:II25-35. doi: 10.1007/pl00007757. J Neurol. 2000. PMID: 10991662 Review.
Cited by
-
The Hidden Notes of Redox Balance in Neurodegenerative Diseases.Antioxidants (Basel). 2022 Jul 26;11(8):1456. doi: 10.3390/antiox11081456. Antioxidants (Basel). 2022. PMID: 35892658 Free PMC article. Review.
-
Moringa oleifera Extracts as Strategic Phyto-Therapy for Alzheimer's Disease.Food Sci Nutr. 2025 Mar 26;13(4):e70007. doi: 10.1002/fsn3.70007. eCollection 2025 Apr. Food Sci Nutr. 2025. PMID: 40144559 Free PMC article.
-
Unveiling the Potential Neuroprotective Effect of Bioactive Compounds from Plants with Sedative and Mood-Modulating Properties: Innovative Approaches for the Prevention of Alzheimer's and Parkinson's Diseases.Curr Neuropharmacol. 2025;23(10):1169-1183. doi: 10.2174/011570159X345397241210103538. Curr Neuropharmacol. 2025. PMID: 39950483 Free PMC article. Review.
-
Metabotropic glutamate receptors and nitric oxide in dopaminergic neurotoxicity.World J Psychiatry. 2021 Oct 19;11(10):830-840. doi: 10.5498/wjp.v11.i10.830. eCollection 2021 Oct 19. World J Psychiatry. 2021. PMID: 34733645 Free PMC article. Review.
-
EAAT3 impedes oligodendrocyte remyelination in chronic cerebral hypoperfusion-induced white matter injury.CNS Neurosci Ther. 2024 Jan;30(1):e14487. doi: 10.1111/cns.14487. Epub 2023 Oct 6. CNS Neurosci Ther. 2024. PMID: 37803915 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

