Bee Venom Melittin Protects against Cisplatin-Induced Acute Kidney Injury in Mice via the Regulation of M2 Macrophage Activation
- PMID: 32899913
- PMCID: PMC7551791
- DOI: 10.3390/toxins12090574
Bee Venom Melittin Protects against Cisplatin-Induced Acute Kidney Injury in Mice via the Regulation of M2 Macrophage Activation
Abstract
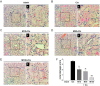
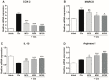
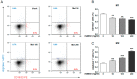
Inflammation is an essential biological response that eliminates pathogenic bacteria and repairs tissue after injury. Acute kidney injury (AKI) is associated with systemic and intrarenal inflammation as the inflammatory process decreases renal function and promotes progression to advanced chronic kidney disease. Macrophages are key mediators of the inflammatory response; their activation influences the immune system and may have various effects. Classically activated type I macrophages (M1) produce a variety of pro-inflammatory cytokines at the lesion site. However, anti-inflammatory type II macrophages (M2) are alternatively activated upon exposure to anti-inflammatory cytokines and are associated with wound healing and tissue repair following AKI. Here, we used melittin from bee venom to enhance the polarization of M2 macrophages and promote renal recovery after AKI. Melittin was administered to mice intraperitoneally for 5 days at various concentrations (10, 50, and 100 µg/kg); serum creatinine and blood urea nitrogen (BUN) levels were analyzed 72 h after cisplatin administration to confirm renal dysfunction. Melittin inhibited the cisplatin-induced increase in creatinine and BUN, an indicator of renal dysfunction. The expression of M1 markers (CD16/32) decreased significantly, whereas that of M2 markers (CD206, Arg1nase I) increased after melittin administration. Consistently, tubular necrosis was substantially reduced in melittin-treated mice. Thus, melittin alleviates cisplatin-induced AKI by regulating M2 macrophage expression.
Keywords: M2 macrophage; acute kidney injury; cisplatin; melittin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
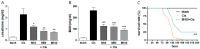
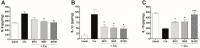


References
-
- Domitrović R., Cvijanovic O., Pernjak-Pugel E., Zagorac G.B., Mahmutefendic H., Škoda M. Luteolin ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of platinum accumulation, inflammation and apoptosis in the kidney. Toxicology. 2013;310:115–123. doi: 10.1016/j.tox.2013.05.015. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

