The Effects of Hypocapnia on Brain Tissue Pulsations
- PMID: 32899967
- PMCID: PMC7565182
- DOI: 10.3390/brainsci10090614
The Effects of Hypocapnia on Brain Tissue Pulsations
Abstract
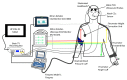
Hypocapnia is known to affect patients with acute stroke and plays a key role in governing cerebral autoregulation. However, the impact of hypocapnia on brain tissue pulsations (BTPs) is relatively unexplored. As BTPs are hypothesised to result from cerebrovascular resistance to the inflow of pulsatile arterial blood, it has also been hypothesised that cerebral autoregulation changes mediated by hypocapnia will alter BTP amplitude. This healthy volunteer study reports measurements of BTPs obtained using transcranial tissue Doppler (TCTD). Thirty participants underwent hyperventilation to induce mild hypocapnia. BTP amplitude, EtCO2, blood pressure, and heart rate were then analysed to explore the impact of hypocapnia on BTP amplitude. Significant changes in BTP amplitude were noted during recovery from hypocapnia, but not during the hyperventilation manoeuvre itself. However, a significant increase in heart rate and pulse pressure and decrease in mean arterial pressure were also observed to accompany hypocapnia, which may have confounded our findings. Whilst further investigation is required, the results of this study provide a starting point for better understanding of the effects of carbon dioxide levels on BTPs. Further research in this area is needed to identify the major physiological drivers of BTPs and quantify their interactions with other aspects of cerebral haemodynamics.
Keywords: BTP; brain tissue pulsations; cerebral autoregulation; hypocapnia.
Conflict of interest statement
M.O. is an employee of Nihon Kohden Corporation (Japan). E.M.L.C. received funding from Nihon Kohden Corporation (Japan), although Nihon Kohden Corporation had no influence on study design, data collection, statistical analysis, or reporting. All other authors declare no conflicts of interest. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research, the Department of Health, or the authors’ respective institutions.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

