Antioxidative Effects of Ascorbic Acid and Astaxanthin on ARPE-19 Cells in an Oxidative Stress Model
- PMID: 32899990
- PMCID: PMC7556018
- DOI: 10.3390/antiox9090833
Antioxidative Effects of Ascorbic Acid and Astaxanthin on ARPE-19 Cells in an Oxidative Stress Model
Abstract
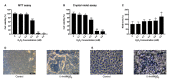
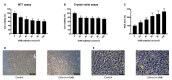
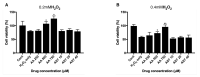
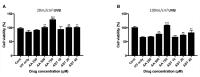
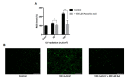
Oxidative stress has been implicated as critical pathogenic factors contributing to the etiology of diabetic retinopathy and other retinal diseases. This study investigated antioxidative effect of ascorbic acid and astaxanthin on ARPE-19 cells within an oxidative stress model induced by common biological sources of reactive oxygen species (ROS). Hydrogen peroxide (H2O2) at concentrations of 0.1-0.8 mM and 20-100 mJ/cm2 of ultraviolet B (UVB) were treated to ARPE-19 cells. Cell viability and intracellular ROS level changes were measured. With the sublethal and lethal dose of each inducers, 0-750 μM of ascorbic acid and 0-40 μM of astaxanthin were treated to examine antioxidative effect on the model. Ascorbic acid at concentrations of 500 and 750 μM increased the cell viability not only in the UVB model but also in the H2O2 model, but 20 and 40 μM of astaxanthin only did so in the UVB model. The combination of ascorbic acid and astaxanthin showed better antioxidative effect compared to each drug alone, suggesting a synergistic effect.
Keywords: antioxidant; ascorbic acid; astaxanthin; diabetic retinopathy; oxidative stress; retinal disease; retinal pigment epithelium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

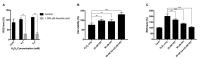
References
-
- Nishikawa M., Inoue M. Oxidative stress and tissue injury. Masui. 2008;57:321–326. - PubMed
-
- Naruse R., Suetsugu M., Terasawa T., Ito K., Hara K., Takebayashi K., Morita K., Aso Y., Inukai T. Oxidative stress and antioxidative potency are closely associated with diabetic retinopathy and nephropathy in patients with type 2 diabetes. Saudi Med. J. 2013;34:135–141. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

