Transfer RNAs: diversity in form and function
- PMID: 32900285
- PMCID: PMC7954030
- DOI: 10.1080/15476286.2020.1809197
Transfer RNAs: diversity in form and function
Abstract
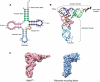
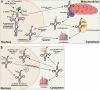
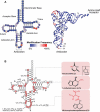
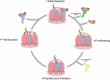
As the adaptor that decodes mRNA sequence into protein, the basic aspects of tRNA structure and function are central to all studies of biology. Yet the complexities of their properties and cellular roles go beyond the view of tRNAs as static participants in protein synthesis. Detailed analyses through more than 60 years of study have revealed tRNAs to be a fascinatingly diverse group of molecules in form and function, impacting cell biology, physiology, disease and synthetic biology. This review analyzes tRNA structure, biosynthesis and function, and includes topics that demonstrate their diversity and growing importance.
Keywords: aminoacylation; mistranslation; tRNA; tRNA fragments; translation.
Figures

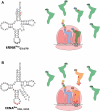
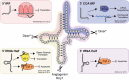
References
-
- Hoagland MB, Stephenson ML, Scott JF, et al. A soluble ribonucleic acid intermediate in protein synthesis. J Biol Chem. 1958;231:241–257. - PubMed
-
- Crick FH. On protein synthesis. Symp Soc Exp Biol. 1958;12:138–163. - PubMed
-
- Crick FHC, Barnett L, Brenner S, et al. General nature of the genetic code for proteins. Nature. 1961;192:1227–1232. - PubMed
-
- Dounce AL. Duplicating mechanism for peptide chain and nucleic acid synthesis. Enzymologia. 1952;15:251–258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
