Tailored Quinolines Demonstrate Flexibility to Exert Antitumor Effects through Varied Mechanisms-A Medicinal Perspective
- PMID: 32900354
- PMCID: PMC9169701
- DOI: 10.2174/1871520620666200908104303
Tailored Quinolines Demonstrate Flexibility to Exert Antitumor Effects through Varied Mechanisms-A Medicinal Perspective
Abstract
Background: Quinoline is considered to be a privileged heterocyclic ring owing to its presence in diverse scaffolds endowed with promising activity profiles. In particular, quinoline containing compounds have exhibited substantial antiproliferative effects through the diverse mechanism of actions, which indicates that the heteroaryl unit is flexible as well as accessible to subtle structural changes that enable its inclusion in chemically distinct anti-tumor constructs.
Methods: Herein, we describe a medicinal chemistry perspective on quinolines as anticancer agents by digging into the peer-reviewed literature as well as patents published in the past few years.
Results: This review will serve as a guiding tool for medicinal chemists and chemical biologists to gain insights about the benefits of quinoline ring installation to tune the chemical architectures for inducing potent anticancer effects.
Conclusion: Quinoline ring containing anticancer agents presents enough optimism and promise in the field of drug discovery to motivate the researchers towards the continued explorations on such scaffolds. It is highly likely that adequate efforts in this direction might yield some potential cancer therapeutics in the future.
Keywords: Quinoline; anticancer; cell line; cytotoxic; heterocycle; medicinal; scaffold.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
Figures





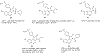

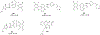
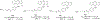
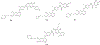
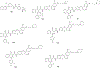

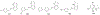
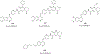
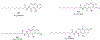


Similar articles
-
Recent contributions of quinolines to antimalarial and anticancer drug discovery research.Eur J Med Chem. 2021 Dec 15;226:113865. doi: 10.1016/j.ejmech.2021.113865. Epub 2021 Sep 23. Eur J Med Chem. 2021. PMID: 34655985 Review.
-
An Overview of Privileged Scaffold: Quinolines and Isoquinolines in Medicinal Chemistry as Anticancer Agents.Curr Top Med Chem. 2020;20(28):2599-2633. doi: 10.2174/1568026620999200917154225. Curr Top Med Chem. 2020. PMID: 32942976
-
Therapeutic significance of quinolines: a patent review (2013-2015).Expert Opin Ther Pat. 2016 Oct;26(10):1201-21. doi: 10.1080/13543776.2016.1216545. Epub 2016 Aug 8. Expert Opin Ther Pat. 2016. PMID: 27458877 Review.
-
Quinoline-derivatives as privileged scaffolds for medicinal and pharmaceutical chemists: A comprehensive review.Chem Biol Drug Des. 2022 Sep;100(3):389-418. doi: 10.1111/cbdd.14099. Epub 2022 Jun 16. Chem Biol Drug Des. 2022. PMID: 35712793 Review.
-
An overview of quinoline as a privileged scaffold in cancer drug discovery.Expert Opin Drug Discov. 2017 Jun;12(6):583-597. doi: 10.1080/17460441.2017.1319357. Epub 2017 Apr 20. Expert Opin Drug Discov. 2017. PMID: 28399679 Review.
Cited by
-
Cancer Cell Inhibiting Sea Cucumber (Holothuria leucospilota) Protein as a Novel Anti-Cancer Drug.Nutrients. 2022 Feb 13;14(4):786. doi: 10.3390/nu14040786. Nutrients. 2022. PMID: 35215436 Free PMC article.
References
-
- Afzal O; Kumar S; Haider MR; Ali MR; Kumar R; Jaggi M; Bawa S A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem, 2015, 97, 871–910. - PubMed
-
- Salat K; Moniczewski A; Librowski T Nitrogen, oxygen or sulfur containing heterocyclic compounds as analgesic drugs used as modulators of the nitroxidative stress. Mini Rev. Med. Chem, 2013, 13, 335–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

