Photoreceptor Discs: Built Like Ectosomes
- PMID: 32900570
- PMCID: PMC7584774
- DOI: 10.1016/j.tcb.2020.08.005
Photoreceptor Discs: Built Like Ectosomes
Abstract
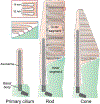
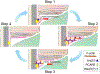
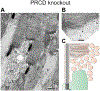
The light-sensitive outer segment organelle of the vertebrate photoreceptor cell is a modified cilium filled with hundreds of flattened 'disc' membranes that provide vast light-absorbing surfaces. The outer segment is constantly renewed with new discs added at its base every day. This continuous process is essential for photoreceptor viability. In this review, we describe recent breakthroughs in the understanding of disc morphogenesis, with a focus on the molecular mechanisms responsible for initiating disc formation from the ciliary membrane. We highlight the discoveries that this mechanism evolved from an innate ciliary process of releasing small extracellular vesicles, or ectosomes, and that both disc formation and ectosome release rely on the actin cytoskeleton.
Keywords: actin cytoskeleton; cilia; extracellular vesicle; outer segment; photoreceptor; vision.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
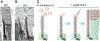


References
-
- Sjostrand FS (1953) The ultrastructure of the outer segments of rods and cones of the eye as revealed by the electron microscope. J Cell Physiol 42 (1), 15–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

