CSPG4-Specific CAR.CIK Lymphocytes as a Novel Therapy for the Treatment of Multiple Soft-Tissue Sarcoma Histotypes
- PMID: 32900797
- PMCID: PMC7710537
- DOI: 10.1158/1078-0432.CCR-20-0357
CSPG4-Specific CAR.CIK Lymphocytes as a Novel Therapy for the Treatment of Multiple Soft-Tissue Sarcoma Histotypes
Abstract
Purpose: No effective therapy is available for unresectable soft-tissue sarcomas (STS). This unmet clinical need prompted us to test whether chondroitin sulfate proteoglycan 4 (CSPG4)-specific chimeric antigen receptor (CAR)-redirected cytokine-induced killer lymphocytes (CAR.CIK) are effective in eliminating tumor cells derived from multiple STS histotypes in vitro and in immunodeficient mice.
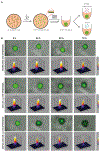
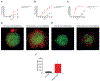
Experimental design: The experimental platform included patient-derived CAR.CIK and cell lines established from multiple STS histotypes. CAR.CIK were transduced with a retroviral vector encoding second-generation CSPG4-specific CAR (CSPG4-CAR) with 4-1BB costimulation. The functional activity of CSPG4-CAR.CIK was explored in vitro, in two- and three-dimensional STS cultures, and in three in vivo STS xenograft models.
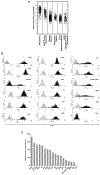
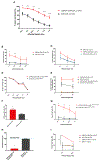
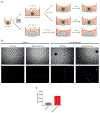
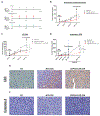
Results: CSPG4-CAR.CIK were efficiently generated from patients with STS. CSPG4 was highly expressed in multiple STS histotypes by in silico analysis and on all 16 STS cell lines tested by flow cytometry. CSPG4-CAR.CIK displayed superior in vitro cytolytic activity against multiple STS histotypes as compared with paired unmodified control CIK. CSPG4-CAR.CIK also showed strong antitumor activity against STS spheroids; this effect was associated with tumor recruitment, infiltration, and matrix penetration. CSPG4-CAR.CIK significantly delayed or reversed tumor growth in vivo in three STS xenograft models (leiomyosarcoma, undifferentiated pleomorphic sarcoma, and fibrosarcoma). Tumor growth inhibition persisted for up to 2 weeks following the last administration of CSPG4-CAR.CIK.
Conclusions: This study has shown that CSPG4-CAR.CIK effectively targets multiple STS histotypes in vitro and in immunodeficient mice. These results provide a strong rationale to translate the novel strategy we have developed into a clinical setting.
©2020 American Association for Cancer Research.
Conflict of interest statement
Figures
Similar articles
-
CSPG4 CAR-redirected Cytokine Induced Killer lymphocytes (CIK) as effective cellular immunotherapy for HLA class I defective melanoma.J Exp Clin Cancer Res. 2023 Nov 22;42(1):310. doi: 10.1186/s13046-023-02884-x. J Exp Clin Cancer Res. 2023. PMID: 37993874 Free PMC article.
-
Cytokine-induced killer cells eradicate bone and soft-tissue sarcomas.Cancer Res. 2014 Jan 1;74(1):119-29. doi: 10.1158/0008-5472.CAN-13-1559. Epub 2013 Dec 19. Cancer Res. 2014. PMID: 24356422
-
CD44v6 as innovative sarcoma target for CAR-redirected CIK cells.Oncoimmunology. 2018 Feb 15;7(5):e1423167. doi: 10.1080/2162402X.2017.1423167. eCollection 2018. Oncoimmunology. 2018. PMID: 29721373 Free PMC article.
-
Adoptive immunotherapy against sarcomas.Expert Opin Biol Ther. 2015 Apr;15(4):517-28. doi: 10.1517/14712598.2015.987121. Epub 2014 Dec 16. Expert Opin Biol Ther. 2015. PMID: 25516119 Review.
-
CSPG4 as Target for CAR-T-Cell Therapy of Various Tumor Entities-Merits and Challenges.Int J Mol Sci. 2019 Nov 26;20(23):5942. doi: 10.3390/ijms20235942. Int J Mol Sci. 2019. PMID: 31779130 Free PMC article. Review.
Cited by
-
Efficacy of CAR-T immunotherapy in MET overexpressing tumors not eligible for anti-MET targeted therapy.J Exp Clin Cancer Res. 2022 Oct 21;41(1):309. doi: 10.1186/s13046-022-02479-y. J Exp Clin Cancer Res. 2022. PMID: 36271379 Free PMC article.
-
CSPG4 CAR-redirected Cytokine Induced Killer lymphocytes (CIK) as effective cellular immunotherapy for HLA class I defective melanoma.J Exp Clin Cancer Res. 2023 Nov 22;42(1):310. doi: 10.1186/s13046-023-02884-x. J Exp Clin Cancer Res. 2023. PMID: 37993874 Free PMC article.
-
Improving Osteosarcoma Treatment: Comparative Oncology in Action.Life (Basel). 2022 Dec 14;12(12):2099. doi: 10.3390/life12122099. Life (Basel). 2022. PMID: 36556464 Free PMC article.
-
Multipurposing CARs: Same engine, different vehicles.Mol Ther. 2022 Apr 6;30(4):1381-1395. doi: 10.1016/j.ymthe.2022.02.012. Epub 2022 Feb 11. Mol Ther. 2022. PMID: 35151842 Free PMC article. Review.
-
CSPG4 Expression in GIST Is Associated with Better Prognosis and Strong Cytotoxic Immune Response.Cancers (Basel). 2022 Mar 3;14(5):1306. doi: 10.3390/cancers14051306. Cancers (Basel). 2022. PMID: 35267618 Free PMC article.
References
-
- Pasquali S, Pizzamiglio S, Touati N, Litiere S, Marreaud S, Kasper B, et al. The impact of chemotherapy on survival of patients with extremity and trunk wall soft tissue sarcoma: revisiting the results of the EORTC-STBSG 62931 randomised trial. Eur J Cancer 2019;109:51–60 doi 10.1016/j.ejca.2018.12.009. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

