Disruption of Adaptive Immunity Enhances Disease in SARS-CoV-2-Infected Syrian Hamsters
- PMID: 32900822
- PMCID: PMC7592214
- DOI: 10.1128/JVI.01683-20
Disruption of Adaptive Immunity Enhances Disease in SARS-CoV-2-Infected Syrian Hamsters
Abstract
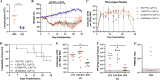
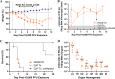
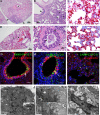
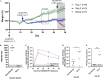
Animal models recapitulating human COVID-19 disease, especially severe disease, are urgently needed to understand pathogenesis and to evaluate candidate vaccines and therapeutics. Here, we develop novel severe-disease animal models for COVID-19 involving disruption of adaptive immunity in Syrian hamsters. Cyclophosphamide (CyP) immunosuppressed or RAG2 knockout (KO) hamsters were exposed to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the respiratory route. Both the CyP-treated and RAG2 KO hamsters developed clinical signs of disease that were more severe than those in immunocompetent hamsters, notably weight loss, viral loads, and fatality (RAG2 KO only). Disease was prolonged in transiently immunosuppressed hamsters and was uniformly lethal in RAG2 KO hamsters. We evaluated the protective efficacy of a neutralizing monoclonal antibody and found that pretreatment, even in immunosuppressed animals, limited infection. Our results suggest that functional B and/or T cells are not only important for the clearance of SARS-CoV-2 but also play an early role in protection from acute disease.IMPORTANCE Syrian hamsters are in use as a model of disease caused by SARS-CoV-2. Pathology is pronounced in the upper and lower respiratory tract, and disease signs and endpoints include weight loss and viral RNA and/or infectious virus in swabs and organs (e.g., lungs). However, a high dose of virus is needed to produce disease, and the disease resolves rapidly. Here, we demonstrate that immunosuppressed hamsters are susceptible to low doses of virus and develop more severe and prolonged disease. We demonstrate the efficacy of a novel neutralizing monoclonal antibody using the cyclophosphamide transient suppression model. Furthermore, we demonstrate that RAG2 knockout hamsters develop severe/fatal disease when exposed to SARS-CoV-2. These immunosuppressed hamster models provide researchers with new tools for evaluating therapies and vaccines and understanding COVID-19 pathogenesis.
Keywords: COVID-19; SARS-CoV-2; Syrian hamster; animal models; cyclophosphamide; disease; monoclonal antibody.
Figures

Similar articles
-
Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development.Proc Natl Acad Sci U S A. 2020 Jul 14;117(28):16587-16595. doi: 10.1073/pnas.2009799117. Epub 2020 Jun 22. Proc Natl Acad Sci U S A. 2020. PMID: 32571934 Free PMC article.
-
Pathogenesis and transmission of SARS-CoV-2 in golden hamsters.Nature. 2020 Jul;583(7818):834-838. doi: 10.1038/s41586-020-2342-5. Epub 2020 May 14. Nature. 2020. PMID: 32408338 Free PMC article.
-
Sex Differences in Lung Imaging and SARS-CoV-2 Antibody Responses in a COVID-19 Golden Syrian Hamster Model.mBio. 2021 Aug 31;12(4):e0097421. doi: 10.1128/mBio.00974-21. Epub 2021 Jul 13. mBio. 2021. PMID: 34253053 Free PMC article.
-
Hamsters as a Model of Severe Acute Respiratory Syndrome Coronavirus-2.Comp Med. 2021 Oct 1;71(5):398-410. doi: 10.30802/AALAS-CM-21-000036. Epub 2021 Sep 29. Comp Med. 2021. PMID: 34588095 Free PMC article. Review.
-
COVID-19: The Immune Responses and Clinical Therapy Candidates.Int J Mol Sci. 2020 Aug 3;21(15):5559. doi: 10.3390/ijms21155559. Int J Mol Sci. 2020. PMID: 32756480 Free PMC article. Review.
Cited by
-
Experimental Animal Models of Coronavirus Infections: Strengths and Limitations.Immune Netw. 2021 Apr 26;21(2):e12. doi: 10.4110/in.2021.21.e12. eCollection 2021 Apr. Immune Netw. 2021. PMID: 33996168 Free PMC article. Review.
-
Animal models for SARS-CoV-2 infection and pathology.MedComm (2020). 2021 Nov 16;2(4):548-568. doi: 10.1002/mco2.98. eCollection 2021 Dec. MedComm (2020). 2021. PMID: 34909757 Free PMC article. Review.
-
SARS-CoV-2 Omicron (B.1.1.529) shows minimal neurotropism in a double-humanized mouse model.Antiviral Res. 2023 Apr;212:105580. doi: 10.1016/j.antiviral.2023.105580. Epub 2023 Mar 20. Antiviral Res. 2023. PMID: 36940916 Free PMC article.
-
Rapid discovery of diverse neutralizing SARS-CoV-2 antibodies from large-scale synthetic phage libraries.MAbs. 2022 Jan-Dec;14(1):2002236. doi: 10.1080/19420862.2021.2002236. MAbs. 2022. PMID: 34967699 Free PMC article.
-
Emerging and reemerging infectious diseases: global trends and new strategies for their prevention and control.Signal Transduct Target Ther. 2024 Sep 11;9(1):223. doi: 10.1038/s41392-024-01917-x. Signal Transduct Target Ther. 2024. PMID: 39256346 Free PMC article. Review.
References
-
- Chan JF, Zhang AJ, Yuan S, Poon VK, Chan CC, Lee AC, Chan WM, Fan Z, Tsoi HW, Wen L, Liang R, Cao J, Chen Y, Tang K, Luo C, Cai JP, Kok KH, Chu H, Chan KH, Sridhar S, Chen Z, Chen H, To KK, Yuen KY. 2020. Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Infect Dis doi:10.1093/cid/ciaa325. - DOI - PMC - PubMed
-
- Rodgers TF, Zhao F, Huang D, Beutler N, Abbott RK, Callaghan S, Garcia E, He W, Hurtado J, Limbo O, Parren M, Peng L, Ricketts J, Ricciardi MK, Smith C, Song G, Woehl J, Yang L, Rawlings S, Smith DM, Nemazee D, Teijaro JR, Voss JE, Andrabi R, Briney B, Landais E, Sok D, Jardine JG, Burton DR. 2020. Rapid isolation of potent SARS-CoV-2 neutralizing antibodies and protection in a small animal model. bioRxiv 10.1101/2020.05.11.088674. - DOI - PMC - PubMed
-
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. doi:10.1016/S0140-6736(20)30183-5. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

