Multiplexed chemogenetics in astrocytes and motoneurons restore blood-spinal cord barrier in ALS
- PMID: 32900826
- PMCID: PMC7479971
- DOI: 10.26508/lsa.201900571
Multiplexed chemogenetics in astrocytes and motoneurons restore blood-spinal cord barrier in ALS
Abstract
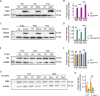
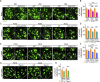
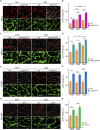
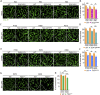
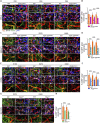
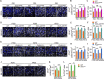
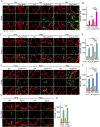
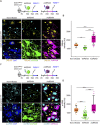
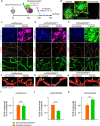
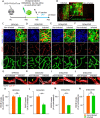
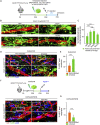
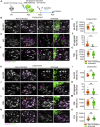
Blood-spinal cord barrier (BSCB) disruption is thought to contribute to motoneuron (MN) loss in amyotrophic lateral sclerosis (ALS). It is currently unclear whether impairment of the BSCB is the cause or consequence of MN dysfunction and whether its restoration may be directly beneficial. We revealed that SOD1 G93A , FUS ΔNLS , TDP43 G298S , and Tbk1 +/- ALS mouse models commonly shared alterations in the BSCB, unrelated to motoneuron loss. We exploit PSAM/PSEM chemogenetics in SOD1 G93A mice to demonstrate that the BSCB is rescued by increased MN firing, whereas inactivation worsens it. Moreover, we use DREADD chemogenetics, alone or in multiplexed form, to show that activation of Gi signaling in astrocytes restores BSCB integrity, independently of MN firing, with no effect on MN disease markers and dissociating them from BSCB disruption. We show that astrocytic levels of the BSCB stabilizers Wnt7a and Wnt5a are decreased in SOD1 G93A mice and strongly enhanced by Gi signaling, although further decreased by MN inactivation. Thus, we demonstrate that BSCB impairment follows MN dysfunction in ALS pathogenesis but can be reversed by Gi-induced expression of astrocytic Wnt5a/7a.
© 2020 Ouali Alami et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


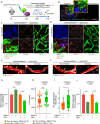


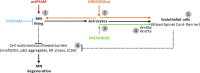
References
-
- Artus C, Glacial F, Ganeshamoorthy K, Ziegler N, Godet M, Guilbert T, Liebner S, Couraud P-O (2014) The Wnt/planar cell polarity signaling pathway contributes to the integrity of tight junctions in brain endothelial cells. J Cereb Blood Flow Metab 34: 433–440. 10.1038/jcbfm.2013.213 - DOI - PMC - PubMed
-
- Aurnhammer C, Haase M, Muether N, Hausl M, Rauschhuber C, Huber I, Nitschko H, Busch U, Sing A, Ehrhardt A (2011) Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum Gene Ther B Methods 23: 18–28. 10.1089/hgtb.2011.034 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
