Cholesterol sensing by CD81 is important for hepatitis C virus entry
- PMID: 32900848
- PMCID: PMC7863897
- DOI: 10.1074/jbc.RA120.014761
Cholesterol sensing by CD81 is important for hepatitis C virus entry
Abstract
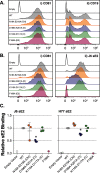
CD81 plays a central role in a variety of physiological and pathological processes. Recent structural analysis of CD81 indicates that it contains an intramembrane cholesterol-binding pocket and that interaction with cholesterol may regulate a conformational switch in the large extracellular domain of CD81. Therefore, CD81 possesses a potential cholesterol-sensing mechanism; however, its relevance for protein function is thus far unknown. In this study we investigate CD81 cholesterol sensing in the context of its activity as a receptor for hepatitis C virus (HCV). Structure-led mutagenesis of the cholesterol-binding pocket reduced CD81-cholesterol association but had disparate effects on HCV entry, both reducing and enhancing CD81 receptor activity. We reasoned that this could be explained by alterations in the consequences of cholesterol binding. To investigate this further we performed molecular dynamic simulations of CD81 with and without cholesterol; this identified a potential allosteric mechanism by which cholesterol binding regulates the conformation of CD81. To test this, we designed further mutations to force CD81 into either the open (cholesterol-unbound) or closed (cholesterol-bound) conformation. The open mutant of CD81 exhibited reduced HCV receptor activity, whereas the closed mutant enhanced activity. These data are consistent with cholesterol sensing switching CD81 between a receptor active and inactive state. CD81 interactome analysis also suggests that conformational switching may modulate the assembly of CD81-partner protein networks. This work furthers our understanding of the molecular mechanism of CD81 cholesterol sensing, how this relates to HCV entry, and CD81's function as a molecular scaffold; these insights are relevant to CD81's varied roles in both health and disease.
Keywords: cholesterol-binding protein; hepatitis C virus (HCV); molecular dynamics; plasma membrane; tetraspanin; virus entry.
© 2020 Palor et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
-
- Kinchen, V. J., Zahid, M. N., Flyak, A. I., Soliman, M. G., Learn, G. H., Wang, S., Davidson, E., Doranz, B. J., Ray, S. C., Cox, A. L., Crowe, J. E., Jr., Bjorkman, P. J., Shaw, G. M., and Bailey, J. R. (2018) Broadly neutralizing antibody mediated clearance of human hepatitis C virus infection. Cell Host Microbe 24, 717–730.e5 10.1016/j.chom.2018.10.012 - DOI - PMC - PubMed
-
- Flyak, A. I., Ruiz, S., Colbert, M. D., Luong, T., Crowe, J. E., Jr., Bailey, J. R., and Bjorkman, P. J. (2018) HCV broadly neutralizing antibodies use a CDRH3 disulfide motif to recognize an E2 glycoprotein site that can be targeted for vaccine design. Cell Host Microbe 24, 703–716.e3 10.1016/j.chom.2018.10.009 - DOI - PMC - PubMed
-
- Banse, P., Moeller, R., Bruening, J., Lasswitz, L., Kahl, S., Khan, A. G., Marcotrigiano, J., Pietschmann, T., and Gerold, G. (2018) CD81 receptor regions outside the large extracellular loop determine hepatitis C virus entry into hepatoma cells. Viruses 10, 207 10.3390/v10040207 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

