Hemolymph protease-5 links the melanization and Toll immune pathways in the tobacco hornworm, Manduca sexta
- PMID: 32900946
- PMCID: PMC7519321
- DOI: 10.1073/pnas.2004761117
Hemolymph protease-5 links the melanization and Toll immune pathways in the tobacco hornworm, Manduca sexta
Abstract
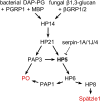
Proteolytic activation of phenoloxidase (PO) and the cytokine Spätzle during immune responses of insects is mediated by a network of hemolymph serine proteases (HPs) and noncatalytic serine protease homologs (SPHs) and inhibited by serpins. However, integration and conservation of the system and its control mechanisms are not fully understood. Here we present biochemical evidence that PO-catalyzed melanin formation, Spätzle-triggered Toll activation, and induced synthesis of antimicrobial peptides are stimulated via hemolymph (serine) protease 5 (HP5) in Manduca sexta Previous studies have demonstrated a protease cascade pathway in which HP14 activates proHP21; HP21 activates proPAP2 and proPAP3, which then activate proPO in the presence of a complex of SPH1 and SPH2. We found that both HP21 and PAP3 activate proHP5 by cleavage at ESDR176*IIGG. HP5 then cleaves proHP6 at a unique site of LDLH112*ILGG. HP6, an ortholog of Drosophila Persephone, activates both proHP8 and proPAP1. HP8 activates proSpätzle-1, whereas PAP1 cleaves and activates proPO. HP5 is inhibited by Manduca sexta serpin-4, serpin-1A, and serpin-1J to regulate its activity. In summary, we have elucidated the physiological roles of HP5, a CLIPB with unique cleavage specificity (cutting after His) that coordinates immune responses in the caterpillar.
Keywords: clip domain; hemolymph protein; insect immunity; serine protease cascade; zymogen activation.
Conflict of interest statement
The authors declare no competing interest.
Figures




Similar articles
-
Inhibition of immune pathway-initiating hemolymph protease-14 by Manduca sexta serpin-12, a conserved mechanism for the regulation of melanization and Toll activation in insects.Insect Biochem Mol Biol. 2020 Jan;116:103261. doi: 10.1016/j.ibmb.2019.103261. Epub 2019 Nov 4. Insect Biochem Mol Biol. 2020. PMID: 31698082 Free PMC article.
-
Manduca sexta hemolymph protease-2 (HP2) activated by HP14 generates prophenoloxidase-activating protease-2 (PAP2) in wandering larvae and pupae.Insect Biochem Mol Biol. 2018 Oct;101:57-65. doi: 10.1016/j.ibmb.2018.08.001. Epub 2018 Aug 8. Insect Biochem Mol Biol. 2018. PMID: 30098411 Free PMC article.
-
Functions of Manduca sexta hemolymph proteinases HP6 and HP8 in two innate immune pathways.J Biol Chem. 2009 Jul 17;284(29):19716-26. doi: 10.1074/jbc.M109.007112. Epub 2009 Jun 1. J Biol Chem. 2009. PMID: 19487692 Free PMC article.
-
Manduca sexta proprophenoloxidase activating proteinase-3 (PAP3) stimulates melanization by activating proPAP3, proSPHs, and proPOs.Insect Biochem Mol Biol. 2014 Jul;50:82-91. doi: 10.1016/j.ibmb.2014.04.005. Epub 2014 Apr 24. Insect Biochem Mol Biol. 2014. PMID: 24768974 Free PMC article.
-
Innate immune responses of a lepidopteran insect, Manduca sexta.Immunol Rev. 2004 Apr;198:97-105. doi: 10.1111/j.0105-2896.2004.0121.x. Immunol Rev. 2004. PMID: 15199957 Review.
Cited by
-
The accumulation of modular serine protease mediated by a novel circRNA sponging miRNA increases Aedes aegypti immunity to fungus.BMC Biol. 2024 Jan 17;22(1):7. doi: 10.1186/s12915-024-01811-6. BMC Biol. 2024. PMID: 38233907 Free PMC article.
-
Rhodnius prolixus uses the peptidoglycan recognition receptor rpPGRP-LC/LA to detect Gram-negative bacteria and activate the IMD pathway.Curr Res Insect Sci. 2020 Dec 13;1:100006. doi: 10.1016/j.cris.2020.100006. eCollection 2021. Curr Res Insect Sci. 2020. PMID: 36003603 Free PMC article.
-
Unveiling the Multifaceted Role of HP6: A Critical Regulator of Humoral Immunity in Antheraea pernyi (Lepidoptera: Saturniidae).Int J Mol Sci. 2025 May 9;26(10):4514. doi: 10.3390/ijms26104514. Int J Mol Sci. 2025. PMID: 40429662 Free PMC article.
-
Parasitoid Serpins Evolve Novel Functions to Manipulate Host Homeostasis.Mol Biol Evol. 2023 Dec 1;40(12):msad269. doi: 10.1093/molbev/msad269. Mol Biol Evol. 2023. PMID: 38061001 Free PMC article.
-
CLIPB4 is a central node in the protease network that regulates humoral immunity in Anopheles gambiae mosquitoes.bioRxiv [Preprint]. 2023 Jul 16:2023.07.07.545904. doi: 10.1101/2023.07.07.545904. bioRxiv. 2023. Update in: J Innate Immun. 2023;15(1):680-696. doi: 10.1159/000533898. PMID: 37461554 Free PMC article. Updated. Preprint.
References
-
- Krem M. M., Di Cera E., Evolution of enzyme cascades from embryonic development to blood coagulation. Trends Biochem. Sci. 27, 67–74 (2002). - PubMed
-
- Kanost M. R., Gorman M. J., “Phenoloxidases in insect immunity” in Insect Immunology, Beckage N., Ed. (Academic Press, San Diego, CA, 2008), pp. 69–96.
-
- Veillard F., Troxler L., Reichhart J. M., Drosophila melanogaster clip-domain serine proteases: Structure, function and regulation. Biochimie 122, 255–269 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

