Photo-mediated selective deconstructive geminal dihalogenation of trisubstituted alkenes
- PMID: 32901002
- PMCID: PMC7479597
- DOI: 10.1038/s41467-020-18274-2
Photo-mediated selective deconstructive geminal dihalogenation of trisubstituted alkenes
Abstract
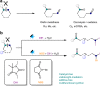
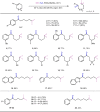
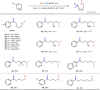
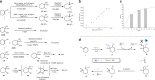
Selective deconstructive functionalization of alkenes, other than the well-established olefin metathesis and ozonolysis, to produce densely functionalized molecular scaffolds is highly attractive but challenging. Here we report an efficient photo-mediated deconstructive germinal dihalogenation of carbon-carbon double bonds. A wide range of geminal diiodoalkanes and bromo(iodo)alkanes (>40 examples) are directly prepared from various trisubstituted alkenes, including both cyclic and acyclic olefins. This C=C cleavage is highly chemoselective and produces geminal dihalide ketones in good yields. Mechanistic investigations suggest a formation of alkyl hypoiodites from benzyl alcohols and N-iodoimides, which undergo light-induced homolytic cleavage to generate active oxygen radical species.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Hoveyda AH, Evans DA, Fu GC. Substrate-directable chemical reactions. Chem. Rev. 1993;93:1307–1370.
-
- Grubbs RH, Chang S. Recent advances in olefin metathesis and its application in organic synthesis. Tetrahedron. 1998;54:4413–4450.
-
- Vasseur A, Bruffaerts J, Marek I. Remote functionalization through alkene isomerization. Nat. Chem. 2016;8:209–219. - PubMed
-
- Dong Z, Ren Z, Thompson SJ, Xu Y, Dong G. Transition-metal-catalyzed C–H alkylation using alkenes. Chem. Rev. 2017;117:9333–9403. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

