IRF2 maintains the stemness of colonic stem cells by limiting physiological stress from interferon
- PMID: 32901054
- PMCID: PMC7479133
- DOI: 10.1038/s41598-020-71633-3
IRF2 maintains the stemness of colonic stem cells by limiting physiological stress from interferon
Abstract
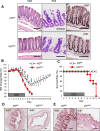
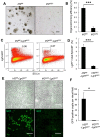
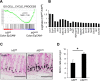
The physiological stresses that diminish tissue stem-cell characteristics remain largely unknown. We previously reported that type I interferon (IFN), which is essential for host antiviral responses, is a physiological stressor for hematopoietic stem cells (HSCs) and small intestinal stem cells (ISCs) and that interferon regulatory factor-2 (IRF2), which attenuates IFN signaling, maintains their stemness. Here, using a dextran sodium sulfate (DSS)-induced colitis model, we explore the role of IRF2 in maintaining colonic epithelial stem cells (CoSCs). In mice with a conditional Irf2 deletion in the intestinal epithelium (hereafter Irf2ΔIEC mice), both the number and the organoid-forming potential of CoSCs were markedly reduced. Consistent with this finding, the ability of Irf2ΔIEC mice to regenerate colon epithelium after inducing colitis was severely impaired, independently of microbial dysbiosis. Mechanistically, CoSCs differentiated prematurely into transit-amplifying (TA) cells in Irf2ΔIEC mice, which might explain their low CoSC counts. A similar phenotype was induced in wild-type mice by repeated injections of low doses of poly(I:C), which induces type I IFN. Collectively, we demonstrated that chronic IFN signaling physiologically stresses CoSCs. This study provides new insight into the development of colitis and molecular mechanisms that maintain functional CoSCs throughout life.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

