The rise and fall of fasciculations in amyotrophic lateral sclerosis
- PMID: 32901231
- PMCID: PMC7425399
- DOI: 10.1093/braincomms/fcaa018
The rise and fall of fasciculations in amyotrophic lateral sclerosis
Abstract
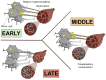
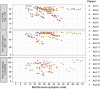
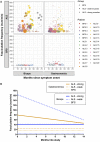
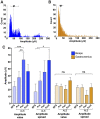
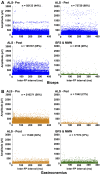
Amyotrophic lateral sclerosis is a devastating neurodegenerative disease with a median survival of 3 years from symptom onset. Accessible and reliable biomarkers of motor neuron decline are urgently needed to quicken the pace of drug discovery. Fasciculations represent an early pathophysiological hallmark of amyotrophic lateral sclerosis and can be reliably detected by high-density surface electromyography. We set out to quantify fasciculation potentials prospectively over 14 months, seeking comparisons with established markers of disease progression. Twenty patients with amyotrophic lateral sclerosis and five patients with benign fasciculation syndrome underwent up to seven assessments each. At each assessment, we performed the amyotrophic lateral sclerosis-functional rating scale, sum power score, slow vital capacity, 30-min high-density surface electromyography recordings from biceps and gastrocnemius and the motor unit number index. We employed the Surface Potential Quantification Engine, which is an automated analytical tool to detect and characterize fasciculations. Linear mixed-effect models were employed to account for the pseudoreplication of serial measurements. The amyotrophic lateral sclerosis-functional rating scale declined by 0.65 points per month (P < 0.0001), 35% slower than average. A total of 526 recordings were analysed. Compared with benign fasciculation syndrome, biceps fasciculation frequency in amyotrophic lateral sclerosis was 10 times greater in strong muscles and 40 times greater in weak muscles. This was coupled with a decline in fasciculation frequency among weak muscles of -7.6/min per month (P = 0.003), demonstrating the rise and fall of fasciculation frequency in biceps muscles. Gastrocnemius behaved differently, whereby strong muscles in amyotrophic lateral sclerosis had fasciculation frequencies five times greater than patients with benign fasciculation syndrome while weak muscles were increased by only 1.5 times. Gastrocnemius demonstrated a significant decline in fasciculation frequency in strong muscles (2.4/min per month, P < 0.0001), which levelled off in weak muscles. Fasciculation amplitude, an easily quantifiable surrogate of the reinnervation process, was highest in the biceps muscles that transitioned from strong to weak during the study. Pooled analysis of >900 000 fasciculations revealed inter-fasciculation intervals <100 ms in the biceps of patients with amyotrophic lateral sclerosis, particularly in strong muscles, consistent with the occurrence of doublets. We hereby present the most comprehensive longitudinal quantification of fasciculation parameters in amyotrophic lateral sclerosis, proposing a unifying model of the interactions between motor unit loss, muscle power and fasciculation frequency. The latter showed promise as a disease biomarker with linear rates of decline in strong gastrocnemius and weak biceps muscles, reflecting the motor unit loss that drives clinical progression.
Keywords: EMG; amyotrophic lateral sclerosis; biomarker; fasciculation; nerve excitability.
Conflict of interest statement
Competing interests The authors report no competing interests.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
