Ultrahigh-resolution MRI Reveals Extensive Cortical Demyelination in a Nonhuman Primate Model of Multiple Sclerosis
- PMID: 32901254
- PMCID: PMC7947170
- DOI: 10.1093/cercor/bhaa235
Ultrahigh-resolution MRI Reveals Extensive Cortical Demyelination in a Nonhuman Primate Model of Multiple Sclerosis
Abstract
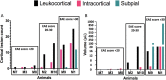
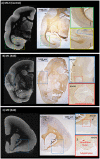
Cortical lesions are a primary driver of disability in multiple sclerosis (MS). However, noninvasive detection of cortical lesions with in vivo magnetic resonance imaging (MRI) remains challenging. Experimental autoimmune encephalomyelitis (EAE) in the common marmoset is a relevant animal model of MS for investigating the pathophysiological mechanisms leading to brain damage. This study aimed to characterize cortical lesions in marmosets with EAE using ultrahigh-field (7 T) MRI and histological analysis. Tissue preparation was optimized to enable the acquisition of high-spatial resolution (50-μm isotropic) T2*-weighted images. A total of 14 animals were scanned in this study, and 70% of the diseased animals presented at least one cortical lesion on postmortem imaging. Cortical lesions identified on MRI were verified with myelin proteolipid protein immunostaining. An optimized T2*-weighted sequence was developed for in vivo imaging and shown to capture 65% of cortical lesions detected postmortem. Immunostaining confirmed extensive demyelination with preserved neuronal somata in several cortical areas of EAE animals. Overall, this study demonstrates the relevance and feasibility of the marmoset EAE model to study cortical lesions, among the most important yet least understood features of MS.
Keywords: EAE; MRI; demyelination; marmoset; multiple sclerosis.
Published by Oxford University Press 2020.
Figures



References
-
- Ascherio A, Munger KL. 2016. Epidemiology of multiple sclerosis: from risk factors to prevention-an update. Semin Neurol. 36:103–114. - PubMed
-
- Bø L, Vedeler CA, Nyland HI, Trapp BD, Mørk SJ. 2003. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol. 62:723–732. - PubMed
-
- Burrows DJ, McGown A, Jain SA, De Felice M, Ramesh TM, Sharrack B, Majid A. 2019. Animal models of multiple sclerosis: from rodents to zebrafish. Mult Scler. 25:306–324. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

