Mannitol Augments the Effects of Systemical Stem Cell Transplantation without Increasing Cell Migration in a Stroke Animal Model
- PMID: 32901436
- PMCID: PMC7524994
- DOI: 10.1007/s13770-020-00293-1
Mannitol Augments the Effects of Systemical Stem Cell Transplantation without Increasing Cell Migration in a Stroke Animal Model
Abstract
Background: Mannitol increases blood-brain barrier permeability and can improve the efficiency of systemically administered stem cells by facilitating stem cell entry from the periphery into the injured brain. The aim of this study was to elucidate the neuroprotective effects of a combination of mannitol pretreatment and stem cell transplantation on stroke-induced neural injury.
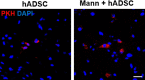
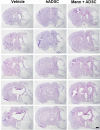
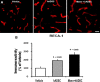
Methods: The experimental rats were randomly assigned to three groups 24 h after middle cerebral artery occlusion and reperfusion. One group received intravenous (IV) injections of phosphate-buffered saline (vehicle), another group received IV injections of human adipose-derived stem cells (hADSCs), and the last group received IV injections of hADSCs 10 min after IV mannitol injections. Neurobehavioral functions and infarct volume were compared. Immunohistochemistry (IHC) analyses were performed using antibodies against ionized calcium binding adapter-1 (IBA-1), rat endothelial antigen-1 (RECA-1), and bromodeoxyuridine/doublecortin (BrdU/DCX).
Results: PKH-26 labeling revealed no difference in the number of stem cells that had migrated into the injured brain, and hADSC transplantation did not improve the infarct volume. However, neurobehavioral functions improved in the mannitol group. IHC showed higher numbers of RECA-1-positive cells in the peri-infarcted brain and BrdU-/DCX-colocalized cells in the subventricular zone in the mannitol group. IBA-1-positive cell number decreased in the hADSC-only and mannitol-pretreatment groups compared with the vehicle group even though there was no difference between the former two groups.
Conclusion: Combinatorial treatment with mannitol and hADSC transplantation may have better therapeutic potential than hADSC monotherapy for ischemic stroke.
Keywords: Combination; Human adipose-derived stem cells; Ischemic stroke; Mannitol; Pretreatment.
Conflict of interest statement
Authors declare there is no conflict of interest with the manuscript.
Figures



References
-
- Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, et al. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke. 2013;44:2361–2375. doi: 10.1161/STR.0b013e31829734f2. - DOI - PubMed
-
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STROKEAHA.118.022606. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
